High-Dose Intravenous Vitamin C Treatment of a Child with Neurofibromatosis Type 1 and Optic Pathway Glioma: A Case Report
Patient: Male, 1
Final Diagnosis: Optic glioma
Symptoms: Visual problems
Medication: —
Clinical Procedure: Intravenous vitamin C
Specialty: Oncology
Authors: Nina Mikirova, Ronald Hunninghake, Ruth C. Scimeca, Charles Chinshaw, Faryal Ali, Chris Brannon, Neil Riordan
Objective: Unusual clinical course
Background: In neurofibromatosis type 1 (NF1) disease, the loss of the tumor suppressor function of the neurofibromin gene leads to proliferation of neural tumors. In children, the most frequently identified tumor is the optic pathway glioma.
Case Report: We describe the case of a 5-year-old child who was diagnosed with NF1 and optic pathway tumor onset at the age of 14 months. Because of the tumor progression, chemotherapy with carboplatin and vincristine was prescribed at this early age and continued for one year. As the progression of disease continued after chemotherapy, the child, at the age of 2.8 years, was started on high-dose intravenous vitamin C (IVC) treatment (7–15 grams per week) for 30 months. After 30 months, the results of IVC treatments demonstrated reduction and stabilization of the tumors in the optic chiasm, hypothalamus, and left optic nerve according to radiographic imaging. The right-sided optic nerve mass seen before IVC treatment disappeared by the end of the treatment.
Conclusions: This case highlights the positive effects of treating NF1 glioma with IVC. Additional studies are necessary to evaluate the role of high-dose IVC in glioma treatment.
MeSH Keywords: Ascorbic Acid • Neurofibromatosis 1 • Optic Nerve Glioma
Full-text PDF: http://www.amjcaserep.com/abstract/index/idArt/899754
Background:
Neurofibromatosis (NF) is an autosomal dominant disorder with a high mutation rate and a prevalence of around 30/100,000 of the population [1]. The National Institutes of Health (NIH) divide neurofibromatosis into type 1 (NF1 or von Recklinghausen syndrome) and type 2 (NF2, acoustic neurofibromatosis, or central neurofibromatosis). The genetic defect in NF1 is usually a mutation in the neurofibromin gene on chromosome 17 (17q11.2). The most common features of the disease include multiple neural tumors (neurofibromas) on the skin or within the body, and pigmented skin lesions.
Neurofibromin normally functions to down-regulate the p21 Ras oncoprotein. The loss of the tumor suppressor function of neurofibromin leads to proliferation of neural tumors. NF1 is a common inherited cancer predisposition syndrome, in which affected individuals are prone to the development of brain tumors [2].
In children, the most frequently identified brain tumor is the optic pathway glioma (OPG) [3,4]. Optic nerve gliomas occur in 12% of patients with NF1, with most of these occurring within the first decade of life and progressing to visual loss or other neurologic symptoms [5].
OPGs are low-grade glial fibrillary acidic protein immunoreactive tumors that typically affect the prechiasmatic optic nerves and chiasm [6,7]. Images exposing optic nerve gliomas may also reveal high signal intensity lesions in the basal ganglia, thalamus, or cerebellum [8, 9], which was the case discussed in the present report.
Determining when to treat young children with NF1-associated OPGs is a challenging clinical problem. As these tumors are grade 1 pilocytic astrocytomas that do not have malignant potential themselves, it has been argued recently that genotoxic chemotherapy and neurotoxic radiotherapy are not indicated in these cases [10].
In cases of progressive disease, traditional radiotherapy is contraindicated and is not recommended now, as it leads to a poorer neuropsychological outcome, secondary tumors, vascular complications, and long-term adverse effects [11,12].
Chemotherapy treatment was introduced in the 1990s to postpone or replace irradiation in the management of children with this type of tumor, and it has become the mainstay treatment for children with progressive OGPs. There have been a number of studies that show visual and radiological stabilization in children with progressing OPG who receive chemotherapy, and a number of phase I and II trials in the United States are looking for new treatments for OPG with new chemotherapy drugs [13,14]. Some studies have shown the efficacy of chemotherapy in inducing tumor response, but administration of chemotherapy can lead to undesirable side effects [15–18]. The accepted drugs for NF1 with optic glioma are carboplatin with vincristine. During carboplatin treatment, 30–40% of patients develop hypersensitivity in the form of rash, and the side effects of vincristine treatment are peripheral (outside the brain and spinal cord) nerve damage.
In 1976 Linus Pauling proposed the treatment of cancer patients with intravenous vitamin C (IVC), followed by oral maintenance. Vitamin C (ascorbic acid, ascorbate), especially at high pharmacological concentrations, has a long and widely discussed history in treating cancer [19–25]. According to our studies and studies by other research groups of high-dose ascorbic acid and cancer [26], there are several advantages in the treatment of such patients with IVC. The IVC therapy is toxic to tumor cells, but not to normal cells; it can suppress angiogenesis and inflammation, boost the immune system, cause differentiation of cells, and improve quality of life of cancer patients.
In this report we document a NF1 optic glioma case treated by IVC after conventional treatment.
Future clinical studies of patients with similar conditions are warranted to prove the benefits of this type of alternative therapy before or after conventional treatment.
Case Report:
A 3-year-old Caucasian male affected by NF1 was brought to the clinic because of a history of chiasm optic glioma. The diagnosis was made at the age of 14 months by magnetic resonance imaging (MRI). At that time, the patient was diagnosed with optic glioma of the left optic nerve and a small right optic glioma. The patient was previously diagnosed with NF1 at three months of age. No other pathological findings were documented at the general physical and neurological examination. Furthermore, due to his age and poor cooperation, the visual function was not evaluated.
Because of the clinical and neuro-radiological progression of the tumor, at the age of 14 months the child started chemotherapy (carboplatin and vincristine), according to the International Society of Pediatric Oncology protocol for progressive low-grade glioma. The treatment consisted of weekly treatment for ten weeks, and then continued with eight cycles consisting of four weeks of chemotherapy with 21 days off during 15 months.
Descriptions of the diagnostic imaging reports during chemotherapy are presented in Table 1. MRI results demonstrated the “continued optic pathway with a slight increase in size and degree of enhancement within hypothalamic component and more prominent contrast enhancement in the left optic nerve.”
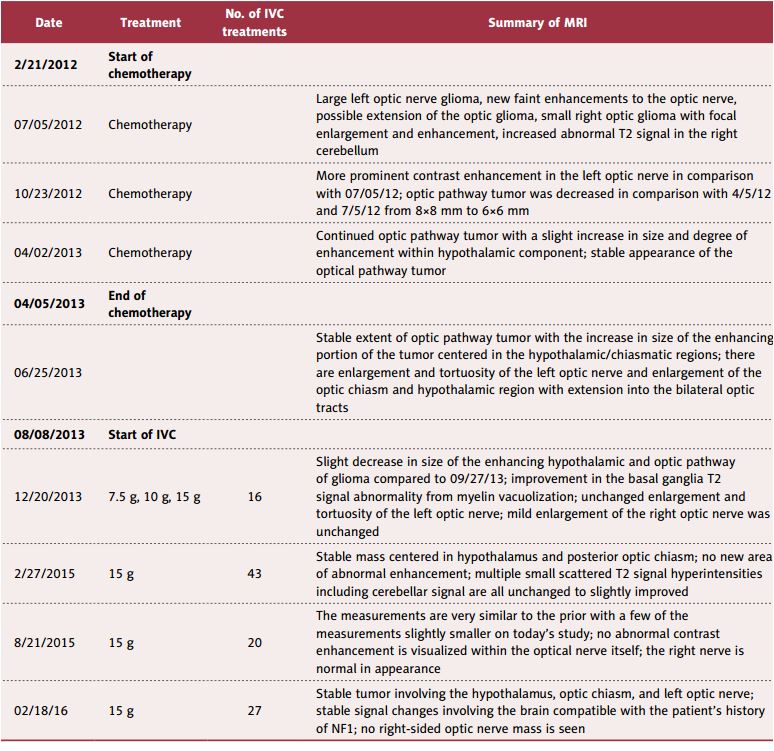
Five months after chemotherapy, on 07/05/2012, the diagnostic MRI reported that the “largest focus within the interior right cerebellum measured 8×8 mm in comparison with 5×3 mm on the prior study in April 2012. There was the marked enlargement of the left optic nerve secondary to the optic glioma, involving the entire length of the optic nerve and extending posteriorly into the orbital apex. The mild interval increased enlargement of the optic chiasm was observed when compared with the prior image. The right optic nerve continued to be mildly enlarged.” The diagnostic images on 10/23/2012 (8 months after chemotherapy) again demonstrated optic pathway tumor with enlargement and tortuosity of the left optic nerve. Abnormal patchy contrast enhancement was presented within the region of the hypothalamus, optic chiasm, and bilateral optic tract. The left optic nerve portion of the optic pathway tumor was decreased in comparison with the images from the beginning of chemotherapy (6×6 mm; previous measurements were 8×9 mm).
The increase of the abnormal T2 bright foci within the bilateral cerebellum and decrease of the left optic portion of optic pathway tumor during chemotherapy are shown in Figure 1.
At the end of the chemotherapy on 04/02/13, MRI demonstrated optic pathway tumor with enlargement and tortuosity of the left optic nerve, and enlargement of the hypothalamus and optic chiasm. The chiasmatic portion appeared slightly more prominent, measuring 19×15×17 mm in comparison with the previously measured 17×11×12 mm. The portion of tumor involving the hypothalamus demonstrated enlargement. There was a more prominent area of enhancement measuring 12×11×9 mm in comparison with the previous size of 4×5×3 mm. During chemotherapy when the patient was 32 months of age, the MRI showed that the size of the tumor centered within the hypothalamic and chiasmatic regions was stable, but the enhancing component of this tumor was increased in size, measuring 2.1×1.9×1.6 cm in comparison with prior measurements of 1.4×1.7×.4 cm.
The examination by the ophthalmologist showed that child did not have any peripheral vision in his left eye. It wasn’t known whether or not he could see with his left eye because he was unable to localize it.
The oncology doctor who had seen the patient was very concerned about the optic pathways, which were torturous on his left eye, extension of the tumor into the chiasm and into the right optic nerve, and the progression of tumor growth. The doctor discussed with the parents additional treatments such as radiation or a different type of chemotherapy that could cause different side effects (nerve problems, ability to walk, hand movement, etc.), but the mother decided to find alternative treatment.
The patient came to the clinic four months after chemotherapy on 08-08-2013. Laboratory tests were performed to evaluate his levels of vitamin C, vitamin D, inflammation (C-reactive protein), and allergic reaction to food by cytotoxic test. Except for a low level of vitamin D (30 ng/mL, normal range 40–80 ng/mL), all other evaluated parameters were in the normal range. The complaints at the first visit were mood swings, poor concentration, night sweats, muscle weakness, impaired vision, teeth grinding, and frequent infections (bronchitis).
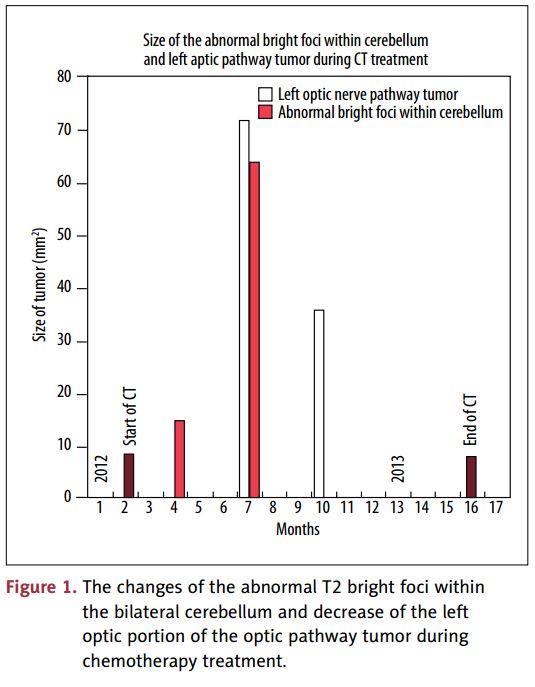
The patient received IVC injections once per week (7 g at the first visit, 10 g the next 4 weeks, and after that 15 g per week, for a total of 100 treatments). All periods of IVC treatment and chemotherapy are shown in Figure 2. The bar shows the duration of the initial chemotherapy, and each square represents the high-dose IVC injection. The values on the X-axis correspond to the amount of injected vitamin C in grams.
Table 1 shows a summary of the MRI reports, the number of IVC treatments between MRIs, and the dosages of injected vitamin C.
The level of ascorbic acid in blood was measured 3 times (after 7 g, 10 g, and 15 g of vitamin C) and reached 190 mg/dL- 210 mg/dL, which was 100 times higher than the pre-treatment level.
After 4 months with a total of 16 IVC treatments, MRI demonstrated that the post-chiasmatic T2 hyperintensity involving the hypothalamus had visually improved with less extension into the bilateral optic pathways. The enhancing portion of this tumor measured 1.8×1.3×1.6 cm in comparison with previous measurements of 2×1.6×2 cm at the beginning of the IVC treatment.
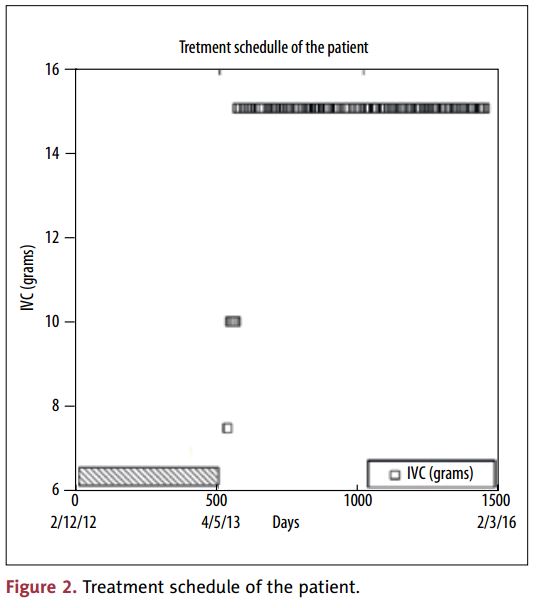
Changes in the hypothalamic component before and during IVC treatment are shown in Figure 3 and demonstrate the reduction in the size of tumor during IVC treatments. According to the data in Figure 3, the size of hypothalamic/optic chiasm tumor had increased about two times at the end of chemotherapy, remained the same size at the beginning of the IVC treatment, and decreased about five times during two years of IVC treatment.
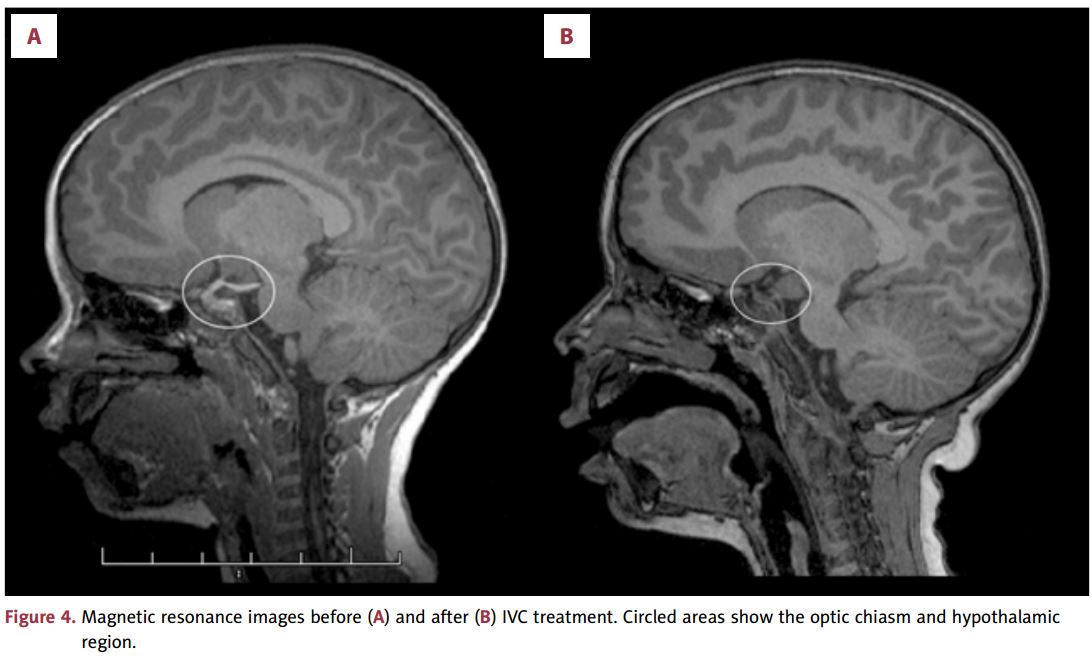
After 2.5 years of the treatment by IVC at the clinic, the patient had an MRI in August 2015 and in February 2016. The results are presented in Table 1 and are shown before and after treatment in Figure 4A and 4B. The MRIs (sagittal view) demonstrate a hyperintense mass (hypothalamic/optic chiasm tumor and optic pathway tumor) before IVC treatment (Figure 4A) and reduced intensity after treatment (Figure 4B). Circled areas in figures indicate the chiasmatic/hypothalamic pathways before and after treatment. The left optic nerve shows hyperintensity on both images with decreased and stable appearance after treatment.
In August 2015 the patient’s oncology doctor stated that the tumor was stable, with shrinkage in the chiasm/hypothalamus area (1.1×1.1×1.0 cm vs. 1.1×1.2×1.3 cm). The tumor on the left optic nerve was still considered enlarged and tortuous.
Half a year later in February 2016 the oncology doctor recommended, based on the results of shrinkage and stability of the tumor over the past two and a half years of IVC treatment, continuation of the treatment and reevaluation in one year. The patient still comes to the clinic weekly and continues highdose IVC treatment. The patient is monitored by our doctors, and there have been no complications caused by the treatment.
Discussion:
We present a case report of a patient with NF1 and optical glioma who had high-dose IVC treatment about three years after one year of conventional treatment (carboplatin and vincristine). To our knowledge it is the first case report of a NF1 patient with OPG treated intravenously by high-dose vitamin C, as an extensive literature review did not reveal cases of children with NF1 and OPG treated by IVC.
The results of the MRI analysis during and at the end of chemotherapy showed the continued optic pathway tumor with a slight increase in size and degree of enhancement within the tumor centered in the hypothalamic/chiasmatic regions.
Approximately 4 months after completion of chemotherapy, the patient started treatment at the Riordan Clinic with highdose IVC (7.5–15 g) one time per week.
As nutritional evaluation of vitamin levels demonstrated low vitamin D, a dose of vitamins D3/K2 5000 IU/50 µg per day was prescribed. At the end of the treatment, the patient’s level of vitamin D increased from 30 ng/mL to 120 ng/mL. Diet improvements were recommended with addition of fruits, vegetables, and a shake supplement with proteins, vitamins, and essential minerals.
The improvement of the patient’s condition after 2.8 years of treatment at the clinic was described based on diagnostic MRI reports in Table 1 and Figures 3 and 4. There was shrinkage/stabilization of the tumors in the hypothalamus, optic chiasm, and left optic nerve according to radiographic imaging. The right optic nerve that showed initial enlargement demonstrated normal appearance according to the last two MRI reports made during IVC treatments.
The patient was treated according to the Riordan Protocol, which involves the slow infusion of high-dose IVC [26]. This protocol is used by many integrative and orthomolecular practitioners for treating a variety of conditions, such as combating infections, treating chronic diseases, and in cancer care.
Our clinic has treated thousands of cancer patients for more than forty years using this protocol. The rationales for using intravenous ascorbate infusions to treat cancer, based on our studies and experience, are summarized in reference 26. According to the studies, ascorbic acid is a key water-soluble antioxidant that, when administered in doses well above its recommended dietary allowance, may have preventative and therapeutic value against a number of pathologies. Ascorbic acid is essential for the formation of collagenase, a basic substance of connective tissue, which may be related to corneal wound healing and prevention of cerebral tissue degeneration [27,28].
In one study, the anti-proliferative and apoptotic effects of ascorbic acid on T98G glioma cells through modulation of IGF-IR expression and consequent facilitation of programmed cell death were reported [29]. A previous study showed upregulation of proteolipid protein (PLP) and myelin-associated glycoprotein (MAG) genes in ascorbic acid–treated rat glioma C6 cells [30].
Our studies have demonstrated that IVC treatment of cancer patients has an anti-inflammatory effect and anti-angiogenic effects [31–33].
It is now shown that ascorbic acid has much a broader impact on the critical stages of tumor cell proliferation and differentiation by shifting their epigenome and transcriptome. For example, nuclear factor erythroid 2-related factor 2 (Nrf2) is an essential component of cellular defense against a variety of endogenous and exogenous stresses. A marked increase in research over the past few decades focusing on Nrf2 and its role in regulating glioblastoma has revealed the potential value of Nrf2 in the treatment of glioblastoma and that ascorbic acid affects the regulation of this factor [34].
Ascorbate plays an important role in regulation of hypoxia-inducible factor 1 (HIF-1a), the dramatic overexpression of which has been observed in common cancers, cancer cell lines, and metastases [35]. Ascorbate has been shown to assist prolyl and lysyl hydroxylases in the hydroxylation of HIF-1a, a transcription factor responsible for the cellular response to low oxygen conditions through activation of genes controlling several cellular transduction pathways, by regulating growth and apoptosis, cell migration, energy metabolism, angiogenesis, and transport of metal ions and glucose [36]. Because HIF-1a prolyl hydroxylase is stimulated by ascorbic acid, low vitamin C levels would reduce HIF-1a hydroxylation and thereby promote HIF-dependent gene transcription and tumor growth [37].
Given the fact that IVC boosts immunity and collagen formation, inhibits hyaluronidase involved in metastasis, induces apoptosis to help program death of cancer cells without harm to normal cells, has anti-inflammatory and anti-angiogenic effects, and improves quality of life of cancer patients, this treatment can be considered as therapy in optic glioma.
In addition to the IVC treatment, supplementation with vitamin D may have a positive effect on the patient’s condition. Vitamin D receptors have important effects not only on physiological processes related to calcium metabolism, but also on cell growth and differentiation. There is in vitro evidence that vitamin D metabolites might be useful agents in the differentiation therapy of human malignant gliomas [38,39]. The major biologically active metabolite of vitamin D has shown a cytotoxic effect on rat and human glioma cells [40].
As scientific evidence suggests that the glioma’s degree of aggressiveness can be modulated by dietary interventions and that some phytochemicals with antioxidant properties and vitamin supplements participate in this process [41–44], the patient was advised on improvement of nutrition and was prescribed supplementation with vitamins and essential minerals.
Conclusions:
In conclusion, in this report we document an optic glioma case associated with NF1 treated by IVC after conventional treatment. This treatment may be recommended for young patients who are not eligible to receive conventional treatment due to its toxicity. Further studies are needed to evaluate the role of high-dose IVC in glioma treatment.
References:
1. Listernick R, Charrow J: Intracranial gliomas in neurofibromatosis type 1. Am J Med Genet, 1999; 89: 38–44
2. Friedman JM, Gutmann DH, MacCollin M et al: Neurofibromatosis: Phenotype, natural history and pathogenesis, 3rd ed., John Hopkins University Press, Baltimore, 1999
3. Listernick R, Charrow J, Greenwald MJ et al: Optic gliomas in children with neurofibromatosis type 1. J Pediatr, 1989; 114: 788–92
4. Listernick R, Louis DN, Packer RJ et al: Optic pathway gliomas in children with neurofibromatosis 1: Consensus statement from the NF1 Optic pathway glioma Task Force. Ann Neurol, 1997; 41: 143–49
5. King A, Listernick R, Charrow J et al: Optic pathway gliomas in neurofibromatosis type 1: The effect of presenting symptoms on outcome. Am J Med Genet A, 2003; 10: 1002
6. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK: WHO Classification of Tumors of the Central Nervous System. Geneva: WHO Press, 2007
7. Listernick R, Ferner RE, Liu GT et al: Optic pathway gliomas in neurofibromatosis-1: Ontroversies and recommendations. Ann Neurol, 2007; 61: 189–98
8. Friedman JM, Gutmann DH, MacCollin M, Riccardi VM: Neurofibromatosis: Phenotype, atural History, and Pathogenesis. 3rd ed. Baltimore, MA: Johns Hopkins University Press, 1999
9. Listernick R, Charrow J, Greenwald MJ et al: Optic gliomas in children with neurofibromatosis type 1. J Pediatr, 1989; 114: 788–92
10. Listernick R, Ferner RE, Liu GT, Gutmann DH: Optic pathway gliomas in neurofibromatosis: Controversies & recommendations. Ann Neurol, 2007; 61: 189–98
11. Grill J, Couanet D, Cappelli C et al: Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol, 1999; 45: 393–96
12. Liu GT: Optic radiation involvement in optic pathway gliomas in neurofibromatosis. Am J Ophthalmol, 2004; 137: 407–14
13. Thiagalingam S, Flaherty M, Billson F, North K: Neurofibromatosis type 1 and optic pathway glioma. Follow up of 54 patients. Ophthalmology, 2004; 111: 568–77
14. Dalla Via P, Opocher E, Pinello ML et al: Visual outcome of a cohort of children with neurofibromatosis 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncology, 2007; 9: 430–37
15. Packer RJ, Lange B, Ater J et al: Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol, 1993; 11: 850–56
16. Packer RJ, Ater J, Allen J et al: Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg, 1997; 86: 747–54
17. Prados MD, Edwards MS, Rabbitt J et al: Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol, 1997; 32: 235–41
18. Gururangan S, Cavazos CM, Ashley D et al: Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol, 2002; 20: 2951–58
19. Cameron E, Campbell A: The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chemico-Biological Interactions, 1974; 9: 285–315
20. Cameron E, Pauling L: Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA, 1976; 73: 3685–89
21. Riordan NH, Riordan HD, Meng X et al: Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses, 1995; 44: 207–13
22. Moertel CG, Fleming TR, Creagan ET et al: High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med, 1985; 312: 137–41
23. Stephenson CM, Levin RD, Spector T, Lis CG: Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol, 2013; 72: 139–46
24. Mikirova N, Casciari J, Riordan N, Hunninghake R: Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. J Transl Med, 2013, 11: 191
25. Riordan HD, Casciari JJ, Gonzalez MJ et al: A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. PR Health Sci J, 2005; 24: 269–76
26. The Riordan Intravenous Vitamin C (IVC) Protocol. Riordan H, Riordan N, Casciari J et al., Book “The Orthomolecular Treatment of Chronic Disease,” Basic Health publication, Saul AW (ed.), 2014; Appendix 3: 750–66
27. Kurata T: Ascorbic acid in: Japan Vitamin Soc ed. Handbook of Vitamin, Water soluble vitamins. Kyoto. Igaku Dojin, 1989; 171–91
28. Rice ME, Lee EJ, Choy Y: High levels of the ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J Neurochem, 1995; 64: 1790–99
29. Naidu KA, Tang JL, Prockop LD et al: Antiproliferative and apoptotic effect of ascorbyl stearate in human glioblastoma multiforme cells: Modulation of insulin-like growth factor-I receptor (IGF-IR) expression. J Neurooncol, 2001; 54: 15–22
30. Laszkiewicz I, Wiggins RC, Konat G: Ascorbic acid upregulates myelin gene expression in C6 glioma cells. Metab Brain Dis, 1992; 7: 157–64
31. Mikirova N, Casciari J Taylor P, Rogers A: Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med, 2012; 10: 189
32. Mikirova N, Riordan N, Casciari J: Modulation of cytokines in cancer patients by intravenous ascorbate therapy. Med Sci Monit, 2016; 22: 14–25
33. Mikirova N, Ichim TE, Riordan NH: Anti-angiogenic effect of high doses of ascorbic acid. J Transl Med, 2008; 6: 50
34. Zhu J, Wang H, Fan Y et al: Targeting the NF-E2-related factor 2 pathway: A novel strategy for glioblastoma (review). Oncol Rep, 2014; 32: 443–50
35. ZhongH, DeMarzo AM, Laughner E et al: Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res, 1999; 59: 5830–35
36. Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol, 2004; 5: 343–54
37. Flashman E, Davies SL, Yeoh KK, Schofield CJ: Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem J, 2010; 427: 135–42
38. Magrassi L, Bono F, Milanesi G, Butti G: Vitamin D receptor expression in human brain tumors. J Neurosurg Sci, 1992; 36: 27–30
39. Magrassi L, Butti G, Pezzotta S et al: Effects of vitamin D and retinoic acid on human glioblastoma cell lines. Acta Neurochir, 1995; 133: 184–90
40. Magrassi L, Adorni L, Montorfano G et al: Vitamin D metabolites activate the sphingomyelin pathway and induce death of glioblastoma cells. Acta Neurochir, 1998; 140: 707–13
41. Pouliquen D, Olivier C, Hervouet E et al: Dietary prevention of malignant glioma aggressiveness, implications in oxidant stress and apoptosis. Int J Cancer, 2008; 123: 288–95
42. Kyritsis AP, Bondy ML, Levin VA: Modulation of glioma risk and progression by dietary nutrients and anti-inflammatory agents. Nutr Cancer, 2011; 63: 174–84
43. Kaplan S, Novikov I, Modan B: Nutritional factors in the etiology of brain tumors: Potential role of nitrosamines, fat, and cholesterol. Am J Epidemiol, 1997; 146: 832–41
44. Tedeschi-Blok N, Lee M, Sison JD et al: Inverse association of antioxidant and phytoestrogen nutrient intake with adult glioma in the San Francisco Bay Area: A case-control study. BMC Cancer, 2006; 6: 148





