Modulation of Cytokines in Cancer Patients by Intravenous Ascorbate Therapy
Modulation of Cytokines in Cancer Patients by
Intravenous Ascorbate Therapy
Corresponding Author: Nina Mikirova, e-mail: nmikirova@riordanclinic.org
Source of support: The study was supported in part by the Flossie E West Memorial Trust
Background: Cytokines play an important role in tumor angiogenesis and inflammation. There is evidence in the literature
that high doses of ascorbate can reduce inflammatory cytokine levels in cancer patients. The objective of this
study was to investigate the effect of treatment by intravenous vitamin C (IVC) on cytokines and tumor markers.
Material/Methods: With the availability of protein array kits allowing assessment of many cytokines in a single sample, we measured 174 cytokines and additional 54 proteins and tumor markers in 12 cancer patients before and after a series of IVC treatments.
Results: Presented results show for our 12 patients the effect of treatment resulted in normalization of many cytokine
levels. Cytokines that were most consistently elevated prior to treatments included M-CSF-R, Leptin, EGF, FGF-6,
TNF-a, b, TARC, MCP-1,4, MIP, IL-4, 10, IL-4, and TGF-b. Cytokine levels tended to decrease during the course of
treatment. These include mitogens (EGF, Fit-3 ligand, HGF, IGF-1, IL-21R) and chemo-attractants (CTAC, Eotaxin,
E-selectin, Lymphotactin, MIP-1, MCP-1, TARC, SDF-1), as well as inflammation and angiogenesis factors (FGF-6,
IL-1b, TGF-1).
Conclusions: We are able to show that average z-scores for several inflammatory and angiogenesis promoting cytokines
are positive, indicating that they are higher than averages for healthy controls, and that their levels decreased
over the course of treatment. In addition, serum concentrations of tumor markers decreased during the time
period of IVC treatment and there were reductions in cMyc and Ras, 2 proteins implicated in being upregulated in cancer.
MeSH Keywords: Allergy and Immunology • Antineoplastic Agents • Ascorbic Acid
Full-text PDF: http://www.medscimonit.com/abstract/index/idArt/895368
Background
Cells produce cytokines in response to a variety of stresses,
including infection, physical trauma, and carcinogen-induced
injury. Cytokines stimulate a coordinated host response aimed
at tissue protection and healing. However, failure of the body
to resolve an injury can lead to persistent cytokine production
and tissue damage. Chronic cytokine production with angiogenesis and inflammation during tumor growth is an example of this. Cancer cells release various cytokines and growth
factors into their surroundings, recruiting and reprogramming other cell types in order to establish a tumor microenvironment [1–11]. Tumor derived cytokines, such as Fas ligand,
vascular endothelial growth factor (VEGF), and transforming
growth factors a and b (TGF), may facilitate the suppression
of immune response to tumors [12,13]. The complex interactions between tumor cells and various other cell types, such
as endothelial cells, fibroblasts, and leukocytes, involve cascades of cytokines and growth factors that in turn can have
local and systemic effects [1–9]. Cytokines such as interleukins 4, 6, 8, and 10 (IL-4, IL-6, IL-8, IL-10) along with TGF and
VEGF, may regulate tumor growth, promote angiogenesis, induce (or inhibit) inflammation, or modify the antitumor immune responses [6–11]. Some cancer patients exhibit chronic
low-grade inflammation that in turn can give rise to fatigue, depression, anorexia, cachexia, pain, and poor prognosis [14,15].
This chronic inflammation is associated with elevated cytokine
levels observed in cancer patients. Interleukins 1, 6, 8, 12, and
13 (IL-1, IL-6, IL-8, IL-12, IL-13), granulocyte macrophage colony-stimulating factor (GM-CSF), monocytes chemoattractant
proteins (MCP-1), macrophage inflammatory proteins (MIP1 a, b), interferon a (IFN-a), tumor necrosis factor (TNF-a), epidermal growth factor (EGF), VEGF, and TNF receptor II have all
been reported to be elevated in cancer patient serum relative
to that of healthy subjects [1–11].
Recent studies suggest that ascorbate (ascorbic acid, vitamin
C) therapy may reduce inflammation and angiogenesis in tumors [16,17].
Intravenous ascorbate therapy is of interest as a potential adjuvant therapy, in part because of the potential for ascorbate,
at sufficient concentrations, to inhibit cancer cell growth while
serving as a biological response modifier. Ascorbate, at concentrations attainable via intravenous infusions, has been shown
to induce apoptosis in cancer cells [18], inhibit tubule formation and angiogenesis [17], stimulate collagen synthesis [19,20],
and reduce the production of pro-inflammatory cytokines [21].
Ascorbate may protect against activation of HIF-1 [22], an inflammation promoter that is upregulated in tumors [23] and
leads to increased production of the angiogenesis factor VEGF.
It also may inhibit the activation of NF-kB, a transcription factor that leads to an overexpression of inflammatory proteins
and cytokines such as IL-2IL-1b and TNF-a [24–28]. Vitamin C
may also inhibit inflammation by blocking the inflammatory
activity of GM-CSF, a cytokine that induces an increase in reactive oxygen species in response to tumor necrosis factor and
IL-1 [29]. The effects of ascorbate on inflammatory cytokines
is concentration dependent, in that doses typical of oral supplementation (250 to 3000 mg per day) do not show any effect [30–35]. Hence, any anti-inflammatory effect of vitamin
C is likely confined to situations where the antioxidant is administered intravenously. Ascorbate may also be important in
supporting immune function, in part through its antioxidant
protection of immune cells [36] and its effects on phosphatase activity, transcription factors, and gene expression [37].
In vitro, T-cell maturation has been shown to depend on vitamin C [38]. Ascorbate may increase phagocytic activity [39] and
have anti-proliferative activity comparable to that of IFN-a [40].
Given the role of cytokines in cancer, and the potential ability
of ascorbate to modulate cytokine production, we undertook
to measure cytokine production in cancer patients before and
after treatment with intravenously administered vitamin C.
The goal of the study was to demonstrate that ascorbate therapy may alter cytokine expression in cancer patients in a way
that is favorable to tumor suppression.
The present manuscript describes measurements of over 170
cytokines in twelve patients with a variety of cancers. In a separate sample of 4 patients, we assayed a panel of over fifty
proteins and cancer markers.
Material and Methods
Serum cytokine and protein analysis
Serum samples from twelve cancer patients and 8 healthy volunteers were obtained for analysis on a voluntary basis with
full HIPAA compliance. The research was in compliance of
the declaration of Helsinki and approved by the Institutional
Review Board of Riordan Clinic. All participants have provided
their written informed consent. The serum concentrations of
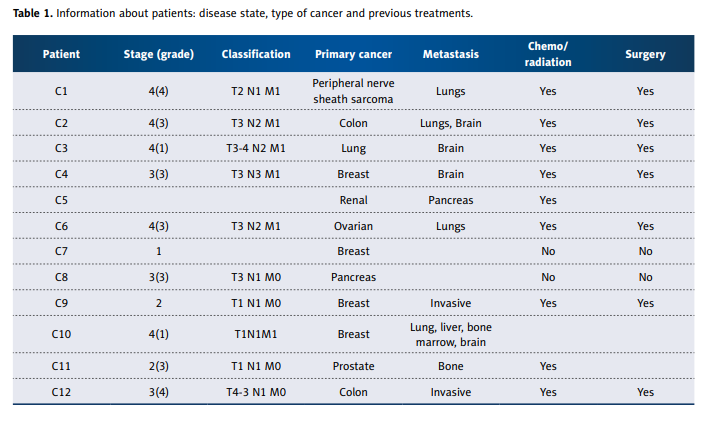
174 cytokines were determined. Information about the cancer patients is summarized in Table1.
Most patients were late stage, had metastatic disease, and
had previously undergone surgery and/or conventional therapy. Cancer patients were treated with intravenous ascorbate infusions at the Riordan Clinic according to the Riordan
IVC protocol. The details of this protocol have been described
elsewhere [41] and are available for download at the Riordan
Clinic web site: https://riordanclinic.org/research-study/vitamin-c-research-ivc-protocol/. Briefly, new cancer patients are
given a 15-gram injection for their first dose, followed by a
25-gram injection the next day. Dosage is then adjusted by
the physician based on the patients’ tolerance and plasma
ascorbate levels attained post infusion. Patients in the present study reached 50 grams per infusion by their sixth course
of treatment. Serum samples for cytokine analysis were taken before the onset of each patient’s first IVC treatment and
after the patient’s sixth IVC treatment. Vitamin C concentrations in plasma immediately after infusions were monitored
using standard protocols [40].
Serum concentrations of 174 cytokines were measured using a protein array kit (AAH-CYT-2000, RayBiotech). The assay
was conducted according to the manufacture’s provided procedure. In this assay, antibodies to 174 cytokines are bound
to a membrane support. After a sample is added and cytokines are allowed to bind to antibodies, additional antibodies
tagged with chemiluminescent markers are added. The chemiluminescent signal was imaged by the BioImage system
(Alpha Innotech). The antigen-antibody spots on the image
were circled by Spot Denso of FluorChem SP software (Alpha
Innotech). Serum samples from 8 healthy volunteers were used
to establish normal ranges. Specifically the mean c
_
and standard deviation sd were determined for each cytokine, with a
±2 SD range being considered “normal”. Z-scores for each cytokine in each cancer patient were computed:
z=(x- mean)/standard deviation
Furthermore, cytokines were also scored based on their ability to promote (+) or inhibit (–) angiogenesis or inflammation.
An angiogenesis score and an inflammation score were then
computed for each sample by taking the sum of the z-scores
for angiogenesis (or inflammation) promotors and subtracting out the z-scores of the angiogenesis (or inflammation) inhibitors. The result was then divided by the total number of
cytokines included in the sum.
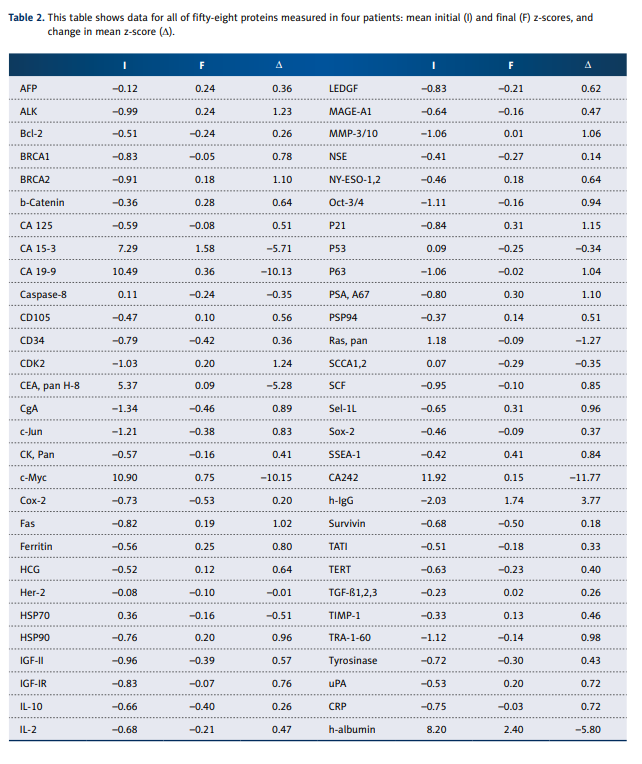
Further tests were done on 4 of 12 cancer patients for IVC influences as measured by protein array assay. Serum concentrations of 58 proteins (a list is provided along with results in
Table 2) were measured by array, which is a label-based assay (with antibody spotted and analyte labeled) for 58 markers of cytokines and oncoproteins based on the principle of
Ray Biotech protein array kit (#AAH-BLM-1-2) and modified.
Serum samples from 8 healthy volunteers (35–60 years old,
4 males and 4 females) were used to establish normal ranges, and z-scores for each protein in each cancer patient were
computed as described above.
Vitamin C in serum of subjects was measured by high-pressure
liquid chromatography (HPLC) with electrochemical detection.
Statistical analysis
The data were analyzed by Systat software (Systat, Inc.) and
Kaleidagraph software. Graphs and curve fits were generated
by Kalaidagraph (Synergy Software) and tests of significance
were carried out using Mann-Whitney non-parametric test.
Variables were presented as mean values ± SD. Statistical
significance was accepted if the null hypothesis could be rejected at p£0.05.
Results
The serum concentrations of 174 cytokines were determined
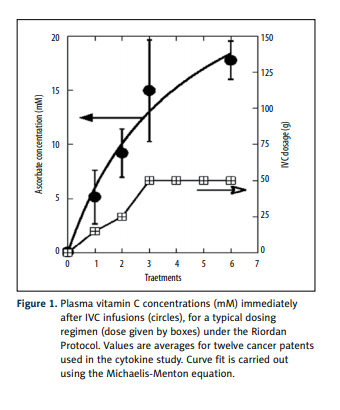
in each of 12 cancer patients before and after a course of intravenous vitamin C therapy. Plasma ascorbate levels, shown
in Figure 1, ranging from 5 mM to 15 mM, were achieved
immediately after infusions, consistent with previous observations with the Riordan protocol [41]. Patients were treated
during 2 weeks by 6 IVC infusions.
The information on the 12 cancer patients is given in Table 1.
Our patients varied considerably in type of cancer, prognosis,
and treatment. Total treatment times ranged from less than
1 month to 52 months, with cancers of the breast, prostate,
colon, lung, sarcoma, and pancreas all represented. Data presented in article were measured at the beginning of patient
enrollment during first 2 weeks.
The comparison of initial samples (prior to therapy) and final samples (after the last of 6 IVC treatments) showed important changes
in cytokine levels. Cytokines that moved in the normal range and
those that moved outside the normal range are listed in Table 3.
The values presented in 2 columns for each patient show the cytokines that were out of normal range (NR ±2SD) and returned
to normal range or were in normal range and decreased or increased to levels lower or higher than NR. Values are given in the
percentage of difference after treatment to pretreatment levels.
Generally, patients with elevated cytokines initially showed the
most dramatic changes over time. The overall effect of treatment was to decrease most cytokine levels; whether this is
due to the ascorbate treatments or simply due to disease progression is not known, since we did not have untreated control subjects in this study, but as the period of treatment was
2 weeks, we suggest that all changes were due to treatment.
There are several cytokines for which a variety of patients saw
decreases after IVC therapy. These include mitogens (EGF, Fit-3
ligand, HGF, IGF-1, IL-21R) and chemo-attractants (CTAC, Eotaxin,
E-selectin, Lymphotactin, MIP-1, MCP-1, TARC, SDF-1), as well
as inflammation and angiogenesis factors (FGF-6, IL-1, TGF-1).
Several improved cytokines were common for several cancer
patients. The cytokines that were increased after treatments
were TIMP (high concentrations are associated with good
prognosis in patients with breast cancer), Beta-NGF (stimulates chemotactic recruitment of leukocytes), I-TAC (potent
chemoattractant for IL-2 activated T cells), IL-1R (antagonistic to IL-1, a non-signaling decoy receptor, which functions by
capturing IL-1 and blocking IL-1R1), and Il-3 (enhances tumor
antigen presentation).
Several cytokines were decreased: MIP-1 (causes local inflammatory response, chemoattractant for T cells and monocytes),
IL-10 (inhibits antigen-presenting cells, inhibits cytokine production), Lymphotactin (enhances T cell recruitment), IL-1 (costimulates cell activation, inflammation, required for tumor
invasion and angiogenesis), BMP (regulates cellular proliferation and apoptosis), and IGF (promotes the proliferation of
many types of cells).
The cytokine profile in these patients was compared to the
normal range (determined from 8 healthy volunteers). We calculated for each patient the percentage of cytokines (from all
measured cytokines) that were in normal range before and after treatment, percentage of cytokines that returned to normal range and percentage of cytokines that remained higher or lower than normal range. The cytokines that remained
in normal range before and after treatment were in a range
of 60–80%, the improved cytokines ranged from 1% to 9%,
with lowest value for the lung cancer patient, and the levels
of 7–9% for patients with prostate, colon, renal, and ovarian
cancers. The cytokines that were not changed and were outside the normal levels ranged from 3% to 23%.
There is quite a contrast, with 7 patients (all breast cancer
patients, sarcoma, lung and colon cancer) having cytokine
levels that are predominately below the normal range while
5 others (prostate, ovarian, pancreas, renal and colon cancers) had cytokine levels that were predominantly in the normal range. Interestingly, all 4 of the breast cancer patients in
our study fell into the first group, showing depressed cytokine levels across the board. Only 28 cytokines had average
z-scores (mean of twelve patients) above zero, with BMP-4,
SDF-1, IL-1alpha, MCP-2 and LIGHT having the highest average z-scores before treatment.
Among the cytokines considered relevant to inflammation
and angiogenesis, the ones with positive mean (for the twelve cancer patients)
z-scores are FGF-4 (0.33), SCF (z_
=0.1), MIP3-alpha (z_
=0.2), IL-9 (z_
=0.24), BMP-6 (z_
=0.54), TGF-beta 3
(z
_
=0.78), CK beta (z_
=1.2), IL-1alpha (z_
=1.8), MIF (z_
=1.04), and
MIP-1-delta (z_
=1.1). All of these cytokines, it should be noticed,
decreased in concentration over the course of IVC therapy.
In an attempt to understand how these changes might affect
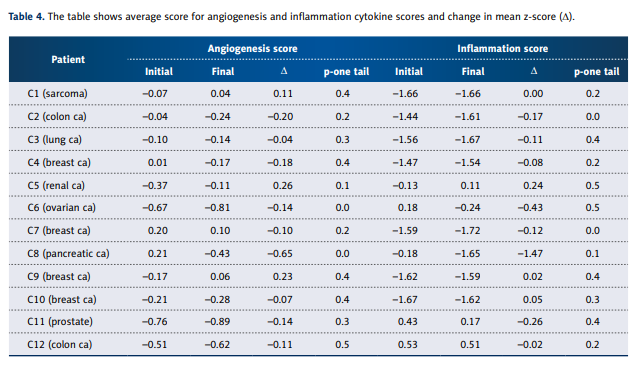
cancer, we separated the angiogenesis factors and inflammation factors. Angiogenesis and inflammation scores for each
patient, computed as described above, are shown in Table 4.
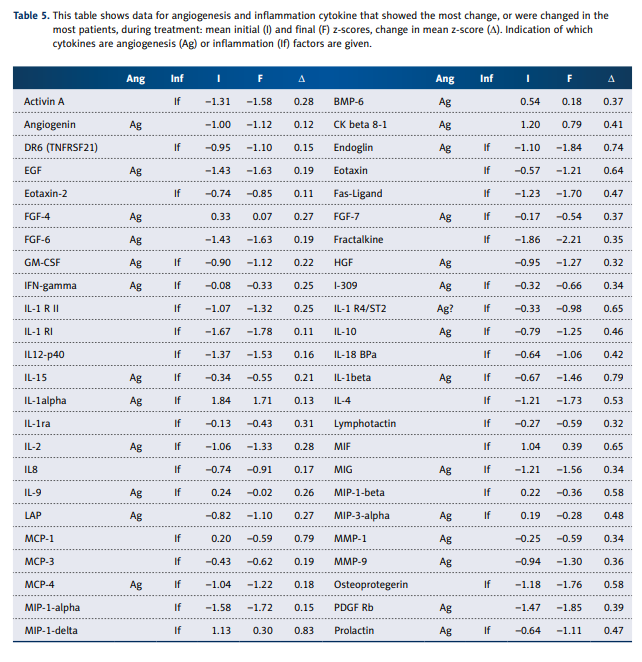
Table 5 shows cytokines from those categories that showed
the most change over the course of treatment.
Some, such as DR6, IL-1a, IL1-R1, and IL-12, showed considerable variability without consistent trends, while others showed
more consistent decreases over time. Particularly striking were
decreases in the angiogenesis factors endoglin, EGF, and FGF-6.
Inflammation factors (or anti-inflammation factors) that decreased most dramatically were eotaxin, IL-4, IL-10, lymphotactin, MCP-1, MIP-1b, TARC, TGF-b3, and TGF- b1. There does
not appear to be any systematic decrease in pro-inflammatory agents versus anti-inflammatory agents.
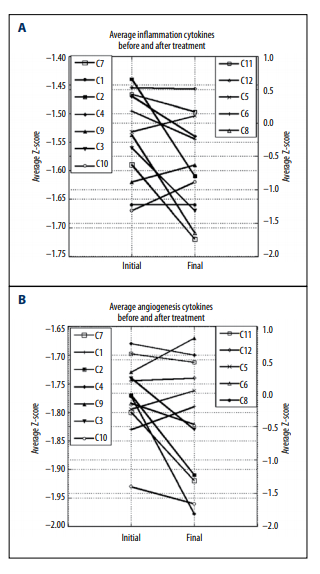
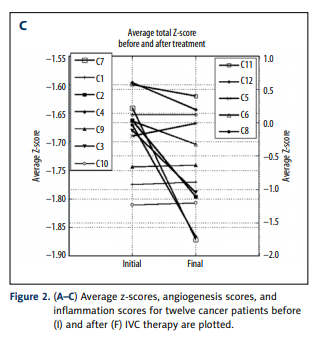
Overall, the inflammation and angiogenesis scores decreased,
suggesting that over time the cytokine mix for these patients’
moves toward a less inflammatory and less angiogenic state.
These trends are shown graphically in Figure 2A–2C. The declines in values are more dramatic in subjects who started with
positive initial values. The changes in z-scores, angiogenesis
scores, and inflammation scores were especially dramatic for
the pancreatic cancer patient, a subject who showed cytokine
profiles characterized by normal to elevated levels before treatment, and reduced levels after treatment. This patient was interesting in that he did not undergo radiation therapy, chemotherapy, or surgery.
Three of these 12 patients had positive results with IVC therapy. Prostate patient had proved refractory to standard treatment and was given a prognosis of death within 1 year; with
IVC treatments he survived for 6 years. The sarcoma patient
began IVC therapy after undergoing radiation and chemotherapy with poor prognosis. She was treated for 3 years, at
which point scans showed her to be clear of almost all metastases. Finally, the breast cancer patient (who had an invasive
tumor with a recurrence score of 23) was treated with IVC after mastectomy. She was treated at the Riordan clinic for 2.5
years before moving to another location. Examining data in
Tables 3 and 5, we do not see much commonality between
these 3 subjects. Patients with breast cancer and sarcoma had
cytokine levels what were reduced during treatment, with large
reductions in angiogenesis and inflammation scores. The patient with prostate cancer, however, showed profiles with reduced cytokine levels and did not show much change in levels during treatment.
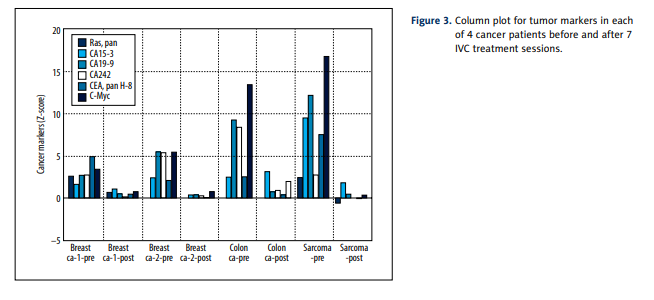
To gain a different perspective, and perhaps narrow the focus a bit, we tested 4 of the 12 cancer patients for serum levels of 58 protein markers. We chose the patient with sarcoma,
who came after radiation and chemotherapy with very poor
prognosis; 2 patients with breast cancer (both after mastectomy, but 1 of them had metastasis and treated by chemotherapy and radiation and another did not have metastasis and
chemotherapy); and the patient with colon cancer after conventional treatment (surgery and chemotherapy). As indicated, these patients were subject to a series of 6 intravenous
vitamin C treatments prior to the final assay. The results for
4 patients of protein profiles and their average z-scores are
listed in Table 2.
Before treatment, all measured proteins except CEA pan H-8,
CA 15-3, h-albumin, CA 19-9, c-Myc and CA242 were in normal
range ±2SD. The most dramatic and consistent results involved
decreases in values of several tumor markers (CA 15-3, CA 19-9,
CEA, and CA 242) as well as decreases in the transcription regulator c-Myc. The gene responsible for producing this protein
is thought to be mutated in cancer cells, leading to increased
expression and increased cell proliferation. Decreases seen
in these markers during treatments are therefore encouraging. Figure 3 shows a histogram to indicate how dramatically
these tumor markers decreased over the course of treatment.
For the average Z-score of Ras protein, we saw a return from
an overproduced state (Z=1.18) to normal ranges (Z=–0.08).
Other encouraging results include statistically significant increases in BRCA1 and BRCA2, both DNA repair agents key in
cell cycle control, and c-Jun, an important factor in cell differentiation, proliferation control, and apoptosis. However, MMP3/10, a matrix metalloproteinase that is critical in maintaining extracellular matrix composition, increased slightly during
treatments.
In summary, the protein assay test shows several tumor markers being reduced or restored to normal ranges over the course
of treatments.
Discussion
We are able to show that average z-scores for several inflammatory and angiogenesis promoting cytokines are positive, indicating that they are higher than averages for healthy controls,
and that their levels decreased over the course of treatment.
Some of these results confirm previous reports, including EGF,
FGF, MIP, MCP, and TNF. Others elevated in our study, including IL-4, 10, MCSF-R, and Leptin, have not been reported previously as being elevated. Of the cytokines that are thought to
be important in angiogenesis and inflammation, we saw dramatic decreases in EGF, FGF, IL-4, IL-10, lymphotactin, MCP-1,
MIP, TARC, TGF-a, and TGF-b. We computed 2 parameters,
the angiogenesis score and the inflammation score, to provide an ‘average’ z-score for cytokines involved in these processes. These scores decreased noticeably during the period
of treatments, with changes being more dramatic in the cancer patients, who had elevated levels of inflammatory and angiogenic cytokines prior to treatment. Our small sample size
did not allow us to demonstrate differences pre- versus posttreatment with 95% confidence, but we did have significance
near the a=0.10 level in some cases, suggesting that further
measurements may be worthwhile.
Our encouraging finding may be the measurements of 58 proteins and markers in 4 cancer patients. We found that serum
concentrations of tumor markers decreased during the time
period of IVC treatment. In addition to the marker decreases, we saw reductions in c-Myc and Ras, 2 proteins implicated in being upregulated in cancer. We also saw increases in
some proteins that might be considered beneficial in combating cancer, including c-Jun, which plays a role in differentiation and apoptosis, and the DNA repair agents BRCA1 and
2. It should be noted that changes that occurred during the
course of therapy (1 or 2 weeks) may be due to the therapy
itself; however, there were no untreated controls for cancer
patients in this study.
The use of high-dose intravenous vitamin C as a possible therapy for cancer has been revisited recently [41–48] and several trails of the use of IVC as adjuvant therapy are under way.
In our study we analyzed cytokines as the messengers for the
regulation of the inflammatory and angiogenic cascades. Our
data confirm the inhibition and positive regulation of the part
of cytokines responsible for angiogenesis and inflammation
after 6 IVC treatments.
Angiogenesis and inflammation share common pathways, and
both play key roles in the initiation and progression of cancer.
Inflammatory cells may facilitate angiogenesis and promote
tumor cell growth, invasion, and metastasis. The FDA has approved several angiogenesis inhibitors for anticancer therapy.
While these drugs seem to have mild adverse effects, recent
studies reveal the potential for complications that reflect the importance of angiogenesis in normal body processes. Research is
also under way to develop pharmaceuticals that reduce inflammation. High-dose ascorbic acid therapy may also be a useful
strategy for reducing inflammation and angiogenesis. Phase I
studies show high-dose (10 to 100 grams) intravenous ascorbate therapy shows a good safety profile [45,49–53] while improving quality of life as measured by cancer patient reported
scores in physical, emotional, and cognitive function. Patients
also show reduced fatigue, nausea/vomiting, pain, and appetite loss after IVC administration.
Conclusions
Because intravenous ascorbate therapy is of interest as a potential adjuvant therapy, we analyzed the levels of cytokines
before and after high-dose ascorbic acid treatment in cancer
patients. The IVC injections resulted in increased concentration of ascorbic acid in blood, from 5 mM after first 15 g IVC
infusion to 15 mM after last 50 g IVC. Given the role of cytokines in cancer, and the potential ability of ascorbate to modulate cytokine production, we measured cytokine production
in cancer patients before and after treatment. It should be
noted that changes that occurred during the time of therapy
(2 weeks) may be considered being due to the therapy itself;
however, there were no untreated controls of cancer patients
in this study. We were able to show that average z-scores for
several inflammatory and angiogenesis promoting cytokines
that were higher than average for healthy controls decreased
over the duration of treatment. In addition, serum concentrations of tumor markers decreased during the period of treatment and there were reductions in c-Myc and Ras proteins
thought to be upregulated in cancer.
In conclusion, the present study suggests that vitamin C therapy can downregulate angiogenesis and inflammation promoting cytokines in some cancer patients. Future studies should
focus on a larger sample of cancer patients, and perhaps a
greater variety of tumor types.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
We are grateful to Meng X, Yin Z, Rogers A, and Zhong J for
their hard work in improvement of cytokine testing procedure
and cytokine measurements.
References:
1. Sheu BC, Chang WC, Cheng CY et al: Cytokine regulation networks in the
cancer microenvironment. Bioscience, 2008; 13: 3152
2. Bruchard M, Ghiringhelli F: Tumor microenvironment: regulatory cells and
immunosuppressive cytokines. Med Sci (Paris), 2014; 30: 429–35
3. Lee S, Margolin K: Cytokines in Cancer Immunotherapy. Cancers, 2005; 3:
3856–93
4. Dranoff G: Cytokines in cancer pathogenesis and cancer therapy. Nat Rev
Cancer, 2004; 4: 11–21
5. Grivennikov SI, Greten FR, Karin M: Immunity, Inflammation and Cancer.
Cell, 2010; 140: 883–99
6. De Simone V, Franzè E, Ronchetti G et al: Th17-type cytokines, IL-6 and
TNF-a synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene, 2015; 34: 3493–503
7. Tarhini AA, Lin Y, Zahoor H, Shuai Y et al: Pro-Inflammatory cytokines predict
relapse-free survival after one month of Interferon-a but not observation
in intermediate risk melanoma patients. PLoS One, 2015; 10: e0132745.
8. Sun X, Ingman WV: Cytokine networks that mediate epithelial cell-macrophage crosstalk in the mammary gland: implications for development and
cancer. J Mammary Gland Biol Neoplasia, 2014; 19: 191–201
9. Gkotzamanidou M, Christoulas D, Souliotis VL et al: Angiogenic cytokines
profile in smoldering multiple myeloma: No difference compared to MGUS
but altered compared to symptomatic myeloma Med Sci Monit, 2013; 19:
1188–94
10. Taniguchi K, Karin M: IL-6 and related cytokines as the critical lynchpins
between inflammation and cancer. Semin Immunol, 2014; 26: 54–74
11. Fukuyama T, Ichiki Y, Yamada S et al: Cytokine production of lung cancer cell
lines: Correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci, 2007; 98: 1048–54
12. Wan-Wan L, Karin M: A cytokine-mediated link between innate immunity,
inflammation, and cancer. J Clin Invest, 2007; 117: 1175–83
13. Kim R, Emi M, Tanabe K, Arihiro K: Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res, 2006; 66:
5527–36
14. Argiles JM, Busquets S, Toledo M et al: The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care, 2009; 3: 263–68
15. Deans C, Wigmore SJ: Systemic inflammation, cachexia and prognosis in
patients with cancer. Curr Opin Clin Nutr Metab Care, 2005; 8: 265–69
16. Mikirova NA, Casciari JJ, Taylor PR, Rogers AM: Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med, 2012;
10: 189
17. Mikirova N, Ichim T, Riordan N: Anti-angiogenic effect of high doses of
ascorbic acid. J Transl Med, 2008; 6: 50
18. Casciari JJ, Riordan NH, Schmidt TS et al: Cytotoxicity of ascorbate, lipoic acid, and other antioxidants in hollow fiber in vitro tumors. Br J Cancer,
2001; 84: 1544–50
19. Qiao H, Bell J, Juliao S et al: Ascorbic acid uptake and regulation of type
I collagen synthesis in cultured vascular smooth muscle cells. J Vasc Res,
2009; 46: 15–24
20. Tsutsumi K, Fujikawa H, Kajikawa T et al: The role of ascorbic acid on collagen structure and levels of serum interleukin-6 and tumour necrosis factor-alpha in experimental lathyrism. J Periodontal Res, 2012; 47: 263–71
21. Hartel C, Strunk T, Bucsky P, Schultz C: Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine, 2004; 27: 101–6
22. Gao P, Zhang H, Dinavahi R et al: HIF-dependent antitumorigenic effect of
antioxidants in vivo. Cancer Cell, 2007; 12: 230–38
23. Kuiper C, Molenaar IG, Dachs GU et al: Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor henotype in endometrial cancer. Cancer Res, 2010; 70: 5749–58
24. Cárcamo JM, Pedraza A, Bórquez-Ojeda O et al: Vitamin C is a kinase inhibitor: dehydroascorbic acid inhibits IkBa kinase b. Mol Cell Biol, 2004;
24: 6645–52
25. Bowie AG, O’Neill LAJ: Vitamin C inhibits NF-kB activation by TNF via the
activation of p38 mitogen-activated protein kinase. J Immunol, 2000; 165:
7180–88
26. Pugazhenthi S, Zhang Y, Bouchard R, Mahaffey G: Induction of an inflammatory loop by interleukin-1b and tumor necrosis factor-a involves NF-kB
and STAT-1 in differentiated human neuroprogenitor cells. Plos One, 2013;
8: e69585
27. Wang DJ, Ratnam NM, Byrd JC, Guttridge DC: NF-kB functions in tumor initiation by suppressing the surveillance of both innate and adaptive immune
cells. Cell Reports, 2014; 9: 90–103
28. Los M, H Schenk, K Hexel et al: IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J, 1995; 14: 3731–40
29. Cárcamo JM, Bórquez-Ojeda O, Golde DW: Vitamin C inhibits granulocyte
macrophage – colony-stimulating factor – induced signaling pathways.
Blood, 1999: 9: 3205
30. Singh J, Singh U: Ascorbic acid has superior ex vivo antiproliferative, cell
death-inducing and immunomodulatory effects over IFN-a in HTLV-1-
associated myelopathy. Am J Clin Nutr, 2006; 83: 525–26
31. Block G, Jensen C, Dietrich M et al: Plasma C-reactive protein concentrations in active and passive smokers: influence of antioxidant supplementation. J Am Coll Nutr, 2004; 23: 141–47
32. Lu Q, Bjorkhem I, Wretlind B et al: Effect of ascorbic acid on microcirculation in patients with type II diabetes: a randomized placebo-controlled
cross-over study. Clin Sci (Lond), 2005; 108: 507–13
33. Fumeron C, Nguyen-Khoa T, Saltiel C et al: Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis
patients. Nephrol Dial Transplant, 2005; 20: 1874–79
34. Bruunsgaard H, Poulsen HE, Pedersen BK et al: Long-term combined supplementations with alphatocopherol and vitamin C have no detectable anti-inflammatory effects in healthy men. J Nutr, 2003; 133: 1170–73
35. Antoniades C, Tousoulis D, Tountas C et al: Vascular endothelium and inflammatory process, in patients with combined type 2 diabetes mellitus
and coronary atherosclerosis: the effects of vitamin C. Diabetes Med, 2004;
21: 552–58
36. Yan Z, Garg SK, Banerjee R: Regulatory T cells interfere with glutathione
metabolism in dendritic cells and T cells. J Biol Chem, 2010; 285: 41525–32
37. Canali R, Natarelli L, Leoni G et al: Vitamin C supplementation modulates
gene expression in peripheral blood mononuclear cells specifically upon an
inflammatory stimulus: a pilot study in healthy subjects. Genes Nutr, 2014;
9: 390–403
38. Manning J, Mitchell B, Appadurai DA et al: Vitamin C promotes maturation
of T-cells. Antioxid Redox Signal, 2013; 19: 2054–67
39. de-la-Fuente M, Ferrandez MD, Burgos MS et al: Immune function in aged
women is improved by ingestion of vitamin C and E. Can J Physiol Pharmacol,
1998; 76: 373–80
40. Moens B, Decanine D, Menezes SM et al: Ascorbic acid has superior ex vivo
antiproliferative, cell death-inducing and immunomodulatory effects over
IFN-a in HTLV-1-Associated Myelopathy. PLoS, 2012; 6: e1729
41. Riordan HD, Riordan N, Casciari J et al: The Riordan Intravenous Vitamin C
(IVC) Protocol for adjunctive cancer care: IVC as a chemotherapeutic and biological response modifying agent. In book: The Orthomolecular Treatment
of Chronic Disease. Basic Health publications, INC, edited by Andrew W.
Saul, 2014
42. Ma Y, Chapman J, Levine M et al: High-dose parenteral ascorbate enhanced
chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy.
Sci Transl Med, 2014; 6: 1–10
43. Yeom CH, Lee G, Park J et al: High dose concentration administration of
ascorbic acid inhibits tumor growth in BLAB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med, 2009;
7: 70
44. Padayatty SJ, Sun AY, Chen Q et al: Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS
One, 2010; 5, e11411
45. Stephenson CM, Levin RD, Spector T, Lis CL: Phase I clinical trial to evaluate
the safety, tolerability,and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol,
2013; 72: 139–46
46. Wilson MK, Baguley BC, Wall C et al: Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac J Clin Oncol, 2014; 10: 22–37
47. Parrow NL, Jonathan A. Leshin, Levine M. Parenteral ascorbate as a cancer
therapeutic: A reassessment based on pharmacokinetics. Antioxid Redox
Signal, 2013; 19: 2141–56
48. Padayatty SJ, Levine M: Reevaluation of ascorbate in cancer treatment:
emerging evidence, open minds and serendipity. J Am Coll Nutr, 2000; 19:
423–25
49. Hoffer LJ, Levine M, Assouline S et al: Phase I clinical trial of i.v. ascorbic
acid in advanced malignancy. Ann Oncol, 2008; 19: 1969–74
50. Monti DA, Mitchell E, Bazzan AJ et al: Phase I evaluation of intravenous
ascorbic acid in combination with gemcitabine and erlotinib in patients
with metastatic pancreatic cancer. PLoS One, 2012; 7: e29794
51. Riordan HD, Casciari JJ, Gonzalez MJ et al: A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J,
2005: 24: 269–76
52. Yeom CH, Jung GC, Song KJ: Changes of terminal cancer patients’ healthrelated quality of life after high dose vitamin C administration. J Korean
Med Sci, 2007; 22: 7–11
53. Takahashi H, Mizuno H, Yanagisawa A: High-dose intravenous vitamin C
improves quality of life in cancer patients. Personalized Medicine Universe,
2012; 1: 49e53