The Riordan Intravenous Vitamin C (IVC) Protocol
THE RIORDAN INTRAVENOUS VITAMIN C (IVC) PROTOCOL FOR
ADJUNCTIVE CANCER CARE: IVC AS A CHEMOTHERAPEUTIC
AND BIOLOGICAL RESPONSE MODIFYING AGENT
by Hugh Riordan, MD, Neil Riordan, PhD, Joseph Casciari, PhD, James Jackson, PhD,
Ron Hunninghake, MD, Nina Mikirova, PhD, and Paul R. Taylor
Grateful appreciation is expressed to the Riordan Clinic for permission to publish their complete intravenous vitamin C protocol.
Vitamin C (ascorbate, ascorbic acid) is a major water-soluble antioxidant that also increases extracellular collagen production and is important for proper immune cell functioning (Hoffman, 1985; Cameron, et al., 1979). It also plays key roles in L-carnitine synthesis, cholesterol metabolism, cytochrome P-450 activity, and neurotransmitter synthesis (Geeraert, 2012). The Riordan intravenous vitamin C (IVC) protocol involves the slow infusion of vitamin C at doses on the order of 0.1 to 1.0 grams (g) of ascorbate per kilogram (kg) body mass (Riordan, et al., 2003). IVC use has increased recently among integrative and orthomolecular medicine practitioners: a survey of roughly 300 practitioners conducted between 2006 and 2008 indicated that roughly 10,000 patients received IVC, at an average dose of 0.5 g/kg, without significant ill effects (Padayatty, et al., 2010). While IVC may have a variety of possible applications, such as combating infections (Padayatty, et al., 2010), treating rheumatoid arthritis (Mikirova, et al., 2012), it has generated the most interest for its potential use in adjunctive cancer care.
Vitamin C was first suggested as a tool for cancer treatment in the 1950s: its role in collagen production and protection led scientists to hypothesize that ascorbate replenishment would protect normal tissue from tumor invasiveness and metastasis (McCormick, 1959; Cameron, et al., 1979). Also, since cancer patients are often depleted of vitamin C (Hoffman, 1985; Riordan, et al., 2005), replenishment may improve immune system function and enhance patient health and well-being (Henson, et al., 1991). Cameron and Pauling observed fourfold survival times in terminal cancer patients treated with intravenous ascorbate infusions followed by oral supplementation (Cameron & Pauling, 1976). However, two randomized clinical trials with oral ascorbate alone conducted by the Mayo Clinic showed no benefit (Creagan, et al., 1979; Moertel, et al., 1985). Most research from that point on focused on intravenous ascorbate. The rationales for using intravenous ascorbate infusions to treat cancer, which are discussed in detail below, can be summarized as follows:
• Plasma ascorbate concentrations in the millimolar (mM) range can be safely achieved with IVC infusions.
• At mM concentrations, ascorbate is preferentially toxic to cancer cells in vitro and is able to inhibit angiogenesis in vitro and in vivo.
• Vitamin C can accumulate in tumors with significant tumor growth inhibition seen (in guinea pigs) at intra-tumor concentrations of 1 mM or higher.
• Published case studies report anticancer efficacy, improved patient well-being, and decreases in markers of inflammation and tumor growth.
• Phase I clinical studies indicate that IVC can be administered safely with relatively few adverse effects.
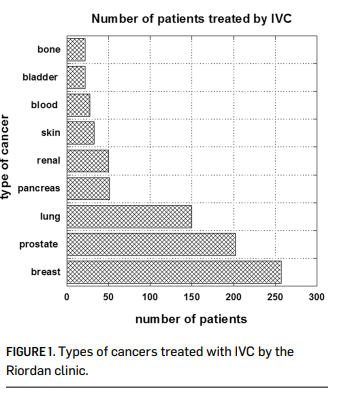
The Riordan Clinic has treated hundreds of cancer patients (Figure 1) using the Riordan protocol. At the same time, Riordan Clinic Research Institute (RCRI) has been researching the potential of intravenous vitamin C therapy for over thirty years. Our efforts have included in vitro studies, animal studies, pharmacokinetic analyses, and
clinical trials. The Riordan IVC protocol, along with the research results (by the RCRI and others) that have motivated its use, is described below.
Scientific Background Pharmacokinetics
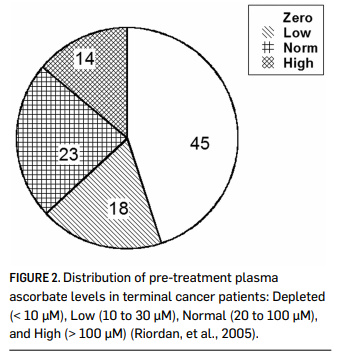
Vitamin C is water soluble, and is limited in how well it can be absorbed when given orally. While ascorbate tends to accumulate in adrenal glands, the brain, and in some white blood cell types, plasma levels stay relatively low (Keith & Pelletier, 1974; Hornig, 1975; Ginter, et al., 1979; Kuether, et al., 1988). Data by Levine and coworkers indicate that plasma levels in healthy adults stayed below 100 micrometer microns (µM), even if 2.5 grams were taken when administered once daily by the oral route. (Levine, et al., 1996.) Cancer patients tend to be depleted of vitamin C: fourteen out of twenty-two terminal cancer patients in a phase I study we depleted of vitamin C, with ten of those having zero detectable ascorbate in their plasma (Riordan, et al., 2005). This is shown in Figure 2. In a study of cancer patients in hospice care, Mayland and coworkers found that thirty percent of the subjects were deficiency in vitamin C (Mayland, et al., 2005). Deficiency (below 10 µM) was correlated with elevated CRP (C-reactive protein, an inflammation marker) levels and shorter survival times. Given the role of vitamin C in collagen production, immune system functioning, and antioxidant protection, it is not surprising that subjects depleted of ascorbate would fare poorly in mounting defenses against cancer. This also suggests that supplementation to replenish vitamin C stores might serve as adjunctive therapy for these patients.
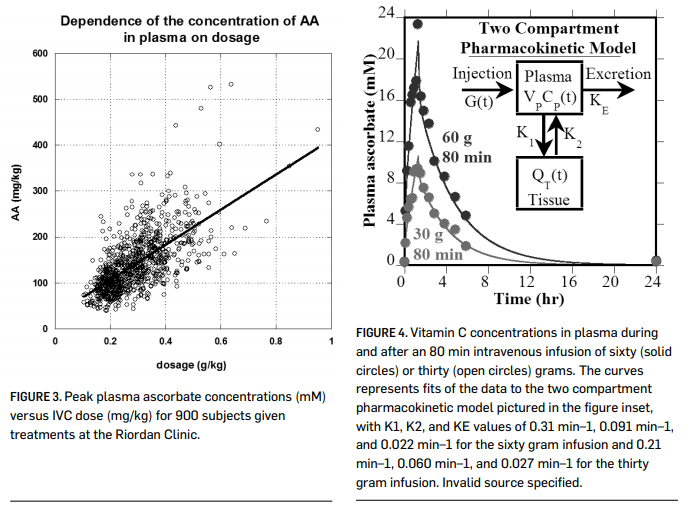
When vitamin C is given by intravenous infusion, peak concentrations over 10 mM can be attained (Casciari, et al., 2001; Padayatty, et al., 2004) without significant adverse effects to the recipient. Figure 3 (page 000) shows plasma ascorbate concentrations attained via IVC infusion at the Riordan Clinic, while Figure 4 shows pharmacokinetic data for two subjects given eighty-minute IVC infusions. These peak plasma concentrations are two orders of magnitude above what is observed with oral supplementation. This suggests that IVC may be more effective than oral supplementation in restoring depleted ascorbate stores in cancer patients. Physicians at the Riordan Clinic have observed that (a) peak plasma concentrations
THE RIORDAN CLINIC INTRAVENOUS VITAMIN C PROTOCOL
AND BACKGROUND RESEARCH
The Riordan Clinic, which focuses on nutritional medicine, was founded in 1975 as the Center for the Improvement of Human Functioning under the leadership of Dr. Hugh Riordan, a medical doctor who practiced psychiatry. Dr. Riordan originally focused on treating mentally ill patients through nutritional medicine. The value of vitamin C as a potential cancer treatment first came into light after an article published in 1976 by Ewan Cameron and Linus Pauling, in which the authors suggested an increased survival in cancer patients receiving intravenous (IV) ascorbate treatments. Dr. Riordan first became aware of this therapy in the early 1980s, when a 70-year-old patient suffering from metastatic renal cell carcinoma that had spread to his liver and lungs came to the clinic requesting IV ascorbate infusions. The patient had read Pauling’s research, and Dr. Riordan was the only doctor in Wichita using IV vitamin C. Upon his request, he began IV vitamin C treatment, starting at 30 grams twice per week. Fifteen months after initial therapy, the patient’s oncologist reported that the patient had no signs of progressive cancer. The patient remained cancer-free for 14 years.
Based on his experience, Dr. Riordan set a goal for a cancer research project. In 1989 Dr. Riordan announced the 11-year RECNAC (cancer spelled backward), a project devoted to finding of the nontoxic cancer treatment. The project manager of the research was Dr. Neil Riordan and the research group included many devoted scientists such as Dr. J. Casciari, Dr. J. Jackson, Dr. X. Meng, Dr. J. Zhong, P. Taylor, BS, Dr. N. Mikirova, and others. The group validated the use of the IV vitamin C for cancer therapy. Using in vitro studies, more than 60 cell lines were tested for the toxicity to high dosages of ascorbate. It was demonstrated that at a high enough dose ascorbate can kill cancer cells while not affecting normal cells. The Riordan Clinic researchers were the first to demonstrate that selectively toxic plasma levels of ascorbate could be achieved in cancer patients (Med Hypothesis, 1995).
In 1997 the IVC treatment was patented by Drs. Neil Riordan and Hugh Riordan; the title of the patent is “Intravenous ascorbate as tumor cytotoxic chemotherapeutic agent.” Other important results included: synergism between vitamin C and alpha-lipoic acid (Br J Cancer, 2001), protocol for reaching tumor cytotoxic blood levels of ascorbate in plasma (P R Health Sci J, 2003), inhibition of the angiogenesis by vitamin C (J Transl Med, 2008), effect of ascorbate on immune function and Phase I trial of the safety of the proposed treatment.
In addition, it was found that IVC treatment improves the quality of life of advanced cancer patients, corrects deficiencies of vitamin C that often occur in cancer patients and optimizes white blood cell concentrations of vitamin C. Analysis of the markers of inflammation in cancer patients showed that high dose intravenous ascorbic acid treatment reduces inflammation in cancer patients. Treatment by IVC can improve response to radiation treatment and reduce the side effects of chemotherapy. In addition, the therapeutic effect of ascorbic acid can be enhanced by vitamin K3 and alpha-lipoic acid. Very important clinical trials inspired by the success of IVC at the Riordan Clinic were conducted at Thomas Jefferson University in Philadelphia. Dr. Hugh Riordan’s vision of treating and preventing ailments with a non-toxic nutritional approach led him to become one of the first doctors to use IV vitamin C as a treatment protocol in patients with terminal cancers. This vision led to original research at the clinic, as well as numerous articles, patents, a series of IVC symposiums, and health initiatives.
attained after IVC infusions tend to be lower in cancer patients than in healthy volunteers, suggesting their depleted tissues act as a “sink” for the vitamin; and (b) in cancer patients given multiple IVC treatments, baseline plasma ascorbate concentrations tend to increase to normal levels slowly over time as reserves are restored with adequate IVC dosing.
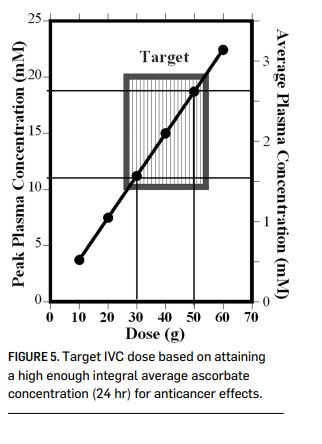
In addition to providing ascorbate replenishment, IVC may allow oncologists to exploit some interesting anticancer properties, including high dose IVC’s ability to induce tumor cell apoptosis, inhibit angiogenesis, and reduce inflammation. In vitro and in vivo data supporting these potential mechanisms of action, discussed below, suggest that they may be relevant at ascorbate concentrations on the order of 2 mM. As shown in Figures 3 and 4 (above), these concentrations are attainable in plasma using progressive dosing of IVC. A 2-compartment model can be used to predict peak and “average” (over 24 hours) plasma ascorbate concentrations for an average-sized adult at a given IVC dose as shown in Figure 5 (page 000). This calculation suggests that a 50 gram, 1 hr. infusion would yield a peak plasma concentration of roughly 18 mM and an integral average of roughly 2.6 mM, a reasonable target for producing anticancer effects.
Peroxide-based Cytotoxicity
Vitamin C, at normal physiological concentrations (0.1 mM), is a major water-soluble antioxidant (Geeraert, 2012). At concentrations on the order of 1 mM, however, continuous perfusion of ascorbate at doses that trigger “redox cycling” can cause a build-up of hydrogen peroxide, which is preferentially toxic toward tumor cells (Benade, et al., 1969; Riordan, et al., 1995; Casciari, et al., 2001; Chen, et al., 2005; Frei & Lawson, 2008), often leading to autophagy or apoptosis.
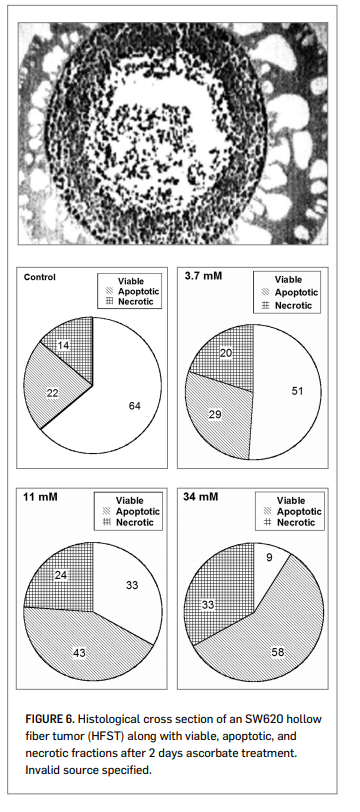
To examine this cytotoxic effect in a three-dimensional model, the RCRI employed hollow-fiber in vitro solid tumors (HFST).
Figure 6 (right) shows a histological section of colon cancer cells growing in this configuration. Dual staining annexin V and propidium iodide flow cytometry showed as significant increase in apoptosis, along with decreased surviving fractions, at ascorbate concentrations in the 1 mM to 10 mM range. Ascorbate concentrations required for toxicity in the HFST model (LC50 = 20 mM), with only two days incubation, were much higher than those typically observed in cell monolayers. The cytotoxic threshold could be reduced significantly (LC50 = 4 mM) by using ascorbate in combination with alpha-lipoic acid. Other reports suggest that ascorbate cytotoxicity against cancer cells
can be increased by using it in combination with menadione (Verrax, et al., 2004) or copper containing compounds (Gonzalez, et al., 2002).
Studies from many laboratories in a variety animal models, using hepatoma, pancreatic cancer, colon cancer, sarcoma, leukemia, prostate cancer, and mesothelioma confirm that ascorbate concentrations sufficient for its cytotoxicity can be attained in vivo, and that treatments can reduce tumor growth (Chen, et al., 2008; Verrax & Calderon, 2009; Belin, et al., 2009; Yeom, et al.,
2009; Du, et al., 2010; Pollard, et al., 2010).
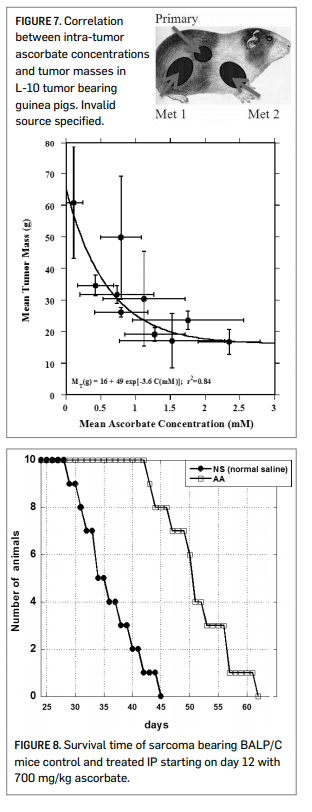
Figure 7 shows data using the L-10 model in guinea pigs. L-10 tumor cells implanted subcutaneously metastasize to the lymph nodes. The overall tumor burden (primary plus metastases) was then determined after 30 days of tumor growth and 18 days of ascorbate therapy. Note that here the actual intra-tumor ascorbate concentrations were measured, and the correlation between tumor mass and tumor ascorbate concentration is strong regardless of the mode of ascorbate administration. The percentage of tumor growth inhibition, relative to controls, was roughly 50% at intra-tumor ascorbate concentrations of 1 mM tumor and roughly 65% once the intra-tumor ascorbate level went above 2 mM. The ascorbate dosage used in this study was 500 mg/kg/day.
Our scientists also looked at survival times of BALP/C mice with S180 sarcomas. The results are shown in Figure 8 (right). The median survival time for the untreated mice was 35.7 days post implantation, while that for ascorbate treated mice (700 mg/kg/day) was 50.7 days. Of course, the efficacy observed in these animal studies may be due to some combination of direct cytotoxicity and other factors, such as angiogenesis inhibition (Yeom, et al., 2009) or other biological response modifications (Cameron, et al., 1979).
Angiogenesis Inhibition
Tumor angiogenesis is the process of new blood vessel growth toward and into a tumor. It is considered to be critical in tumor growth and metastasis. Reports in the literature suggest that ascorbate’s effect on collagen synthesis can act to inhibit formation of new vascular tubules (Ashino, et al., 2003), that ascorbate can inhibit genes
necessary for angiogenesis (Berlin, et al., 2009), and that it might influence angiogenesis through its effect on hypoxia induceable factor (Page, et al., 2007).
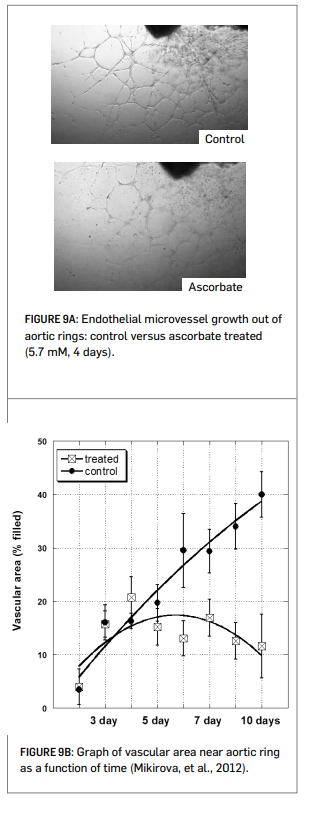
The Riordan Clinic researchers evaluated angiogenesis inhibition using four different experimental models. In all cases, there is an inhibitory effect on angiogenesis at ascorbate concentrations of 1 to 10 mM (Mikirova, et al., 2008; Mikirova, et al, 2012; see Figures 9A and 9B right).
• The growth of new micro-vessels from aortic rings ex vivo is inhibited by ascorbate at concentrations of 5 mM of more.
• Ascorbate inhibits endothelial cell tubule formation in Matrigel in vitro in a concentration-dependent fashion. Number of intact tubule loops was decreased by half at concentrations of 11 mM for
endothelial progenitor cells and 17 mM for HUVEC cells.
• The rate at which endothelial cells can migrate on a petri dish to fill a gap between them was reduced when 5.7 mM ascorbate was added after the gap was created. The ascorbate also reduced ATP production in these endothelial cells by twenty percent, but did not affect cell viability.
• For Matrigel plugs implanted subcutaneously in mice, the micro-vessel density we significantly lower in mice treated with 430 mg/kg every other day for two weeks.
In animal experiments and clinical case studies where high ascorbate doses show efficacy against tumors, this benefit may represent therapeutic synergism due to both angiogenesis inhibition as well as to direct cytotoxicity or other causes.
Inflammation Modulation
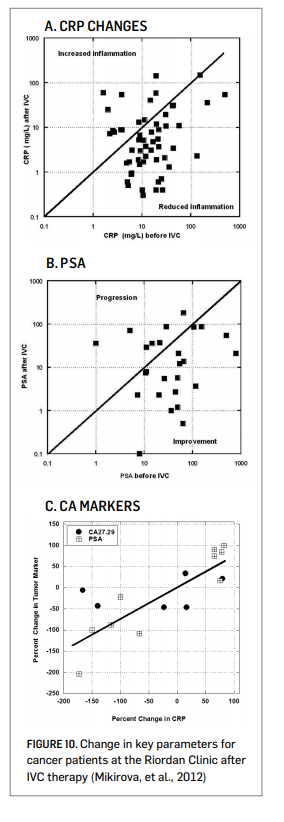
Analysis of clinical data from the Riordan Clinic suggests that inflammation is an issue for cancer patients, and that it can be lessened during IVC therapy (Mikirova, et al., 2012). C-reactive protein was used as a marker of inflammation, as reports in the literature indicate that elevated CRP is correlated with poor patient prognosis (St. Sauver, et al., 2009). Over sixty percent of analyzed Riordan Clinic cancer patients had CRP levels above 10 mg/L prior
to IVC therapy. In 76 ± 13% of these subjects, IVC reduced CRP levels. This improvement was more prevalent, 86 ± 13%, in subjects with elevated (above 10 mg/L) CRP. Comparisons of individual values before and after treatments are shown in Figure 10A (right).
Since many of the subjects in this database were prostate cancer patients, we examined prostate specific antigen (PSA) levels before and after therapy. This is shown in Figure 10B (right). Most of the prostate cancer patients showed reductions in PSA levels during the course of their IVC therapy. This was not true with other markers, as shown in Figure 10C (right). In some subjects, both tumor marker and CRP data were available both before and after IVC therapy. In those cases, there was a strong correlation (r2 = 0.62) between the change in tumor marker and the change in CRP during IVC therapy. This is consistent with observations from the literature showing a correlation between CRP levels and PSA levels in prostate cancer patients (Lin, et al., 2010). The potential effect of IVC in reducing inflammation is also supported by cytokine data: serum concentrations of the pro-inflammatory cytokines IL-1α, IFN-γ, IL-8, IL-2, TNF-α and eotaxin were acutely reduced after a 50-gram ascorbate infusion, and in the case of the last three cytokines listed, reductions were maintained throughout the course of IVC therapy (Mikirova, et al., 2012).
Chemotherapy Controversy
The observations that ascorbate is an antioxidant and that it preferentially accumulates in tumors (Agus, et al., 1999) have raised fears that ascorbate supplementation would compromise the efficacy of chemotherapy (Raloff, 2000). In support of this, Heaney and coworkers found that tumor cells in vitro and xenografts in mice were more resistant to a variety of anticancer agents when the tumor cells were pretreated with dehydroascorbic acid (Heaney, et al., 2008).
Questions have been raised, however, whether the experimental conditions used in the Heaney study are clinically or biochemically relevant, considering, among other issues, that dehydroascorbic acid rather than ascorbic acid was used (Espey, et al., 2009). It should also be noted that the goal of IVC is to attain mM intra-tumor concentrations (for the reasons described above) and thus the accumulation of ascorbate in tumors is considered an advantage.
A variety of laboratory studies suggest that, at high concentrations, ascorbate does not interfere with chemotherapy or irradiation and may enhance efficacy in some situations (Fujita, et al., 1982; Okunieff & Suit, 1987; Kurbacher, et al., 1996; Taper, et al., 1996; Fromberg, et al., 2011; Shinozaki, et al., 2011; Espey, et al., 2011). This is supported by meta-analyses of clinical studies involving cancer and vitamins; these studies conclude that antioxidant supplementation does not interfere with the toxicity of chemotherapeutic regimens (Simone, et al., 2007; Block, et al., 2008).
Clinical Data Case Studies
The situation with intravenous ascorbate therapy is different from that with new chemotherapeutic agents in that Food and Drug Administration (FDA) approval was not strictly required in order for physicians to administer IVC. As a result, clinical investigations tended to run concurrently with laboratory research. Two early studies indicated that intravenous ascorbate therapy could increase survival times beyond expectations in cancer patients (Cameron & Pauling, 1976; Murata, et al., 1982). There have been several case studies published by the Riordan Clinic team (Jackson, et al., 1995; Riordan, et al., 1996; Riordan, et al., 1998) and collaborators (Drisko, et al., 2003; Padayatti, et al., 2006).
While these case studies do not represent conclusive evidence in the same way that a well-designed Phase III study would, they are nonetheless of interest for comparing methodologies and motivating future research, in addition to being of monumental importance to the individuals who were their subjects. Some key case studies are summarized here:
A.A 51-year-old female with renal cell carcinoma (nuclear grade III/IV) and lung metastasis declined chemotherapy and instead chose to intravenous ascorbate at an initial dose of 15 grams. Her dose was increased to 65 grams after two weeks. She continued at this dose for ten months. Patient received no radiation or chemotherapy. The patient supplemented with thymus protein extract, N-acetylcysteine, niacinamide, beta-glucan, and thyroid extract. Seven of eight lung masses resolved. Patient went four years without evidence of regression. Four years later, patient showed a new mass (consistent with small-cell lung cancer, not with recurrent renal carcinoma metastasis) and died shortly afterward (Padayatti, et al., 2006).
B. A 49-year-old male with a bladder tumor (invasive grade 3/3 papillary transitional cell carcinoma) and multiple satellite tumors declined chemotherapy and instead chose to receive intravenous ascorbate. He received 30 grams twice weekly for three months, followed by 30 grams monthly for four years. Patient supplementation included botanical extract, chondroitin sulfate, chromium picolinate, flax oil, glucosamine sulfate, alpha-lipoic acid, lactobacillus acidophilus, L. rhamnosus, and selenium. Nine years after the onset of therapy, patient is in good health with no signs of recurrence or metastasis (Padayatti, et al., 2006).
C.A 66-year-old woman with diffuse Stage III large B-cell lymphoma with a brisk mitotic rate and large left paraspinal mass (3.5–7 cm transverse and 11 cm craniocaudal) showing evidence of bone invasion agreed to a five-week course of radiation therapy, but refused chemotherapy and instead chose to receive intravenous ascorbate concurrent with radiation. She received 15 grams twice weekly for two months, once per week for seven months, and then once every two-three months for one year. Patient supplementation included coenzyme Q10, magnesium, beta-carotene, parasidal, vitamin B and C supplements, Parex, and Nacetylcysteine. The original mass remained palpable after radiation therapy and a new mass appeared. Vitamin C therapy continued. Six weeks later, masses were not palpable. A new lymph mass was detected after four months, but the patient showed no clinical signs of lymphoma after one year. Ten years diagnosis, the patient remained in normal health (Padayatti, et al., 2006).
D. A 55-year-old woman with stage IIIC papillary adenocarcinoma of the ovary and an initial CA-125 of 999 underwent surgery followed by six cycles of chemotherapy (Paclitaxel, Paraplatin) combined with oral and parenteral ascorbate. Ascorbate infusion began at 15 grams twice weekly and increased to 60 grams twice weekly. Plasma ascorbate levels above 200 mg/dL were achieved during infusion. After six weeks, ascorbate treatment continued for one year, after which patient reduced infusions to once every two weeks. The patient also supplemented with vitamin E, coenzyme Q10, vitamin C, beta-carotene, and vitamin A. At the time of publication, she was over 40 months from initial diagnosis and remained on ascorbate infusions. All computed tomography (CT) and positron emission tomography (PET) scans were negative for disease, and her CA-125 levels remained normal (Drisko, et al., 2003).
E. A 60-year-old woman with stage IIIC adenocarcinoma of the ovary and an initial CA-125 of 81 underwent surgery followed by six cycles of chemotherapy (paclitaxel, carboplatin) with oral antioxidants. After six cycles of chemotherapy, patient began parenteral ascorbate infusions. Ascorbate infusion began at 15 grams once weekly and increased to 60 grams twice weekly. Plasma ascorbate levels above 200 mg/dL were achieved during infusion. Treatment continued to date of publication. The patient supplemented with vitamin E, coenzyme Q10, vitamin C, beta-carotene, and vitamin A. Her CA-125 levels normalized after one course of chemotherapy. After the first cycle of chemotherapy, the patient was noted to have residual disease in the pelvis. At this point, she opted for intravenous ascorbate. Thirty months later, patient showed no evidence of recurrent disease and her CA-125 levels remained normal.
Note that these case studies involve a variety of cancer types, sometimes involve the use of IVC in conjunction with chemotherapy or irradiation, and usually involve the use of other nutritional supplements by the subject.
Several other clinical studies looked into the effect of vitamin C on quality of life in cancer patients.
In a Korean study, IVC therapy significantly improved global quality of life scores, with benefits including less fatigue, reduction in nausea and vomiting, and improved appetite (Yeom, et al., 2007).
In a recent German study, breast cancer patients receiving IVC along with standard therapy were compared to subjects receiving standard therapy alone (Vollbracht, et al., 2011). Patients
given IVC benefited from less fatigue, reduction in nausea, improved appetite, reductions in depression and fewer sleep disorders. Overall intensity scores of symptoms during therapy and aftercare were twice as high in the control group as the IVC group. No side effects due to ascorbate were observed, nor were changes in tumor status compared to controls reported.
Phase I Clinical Trials
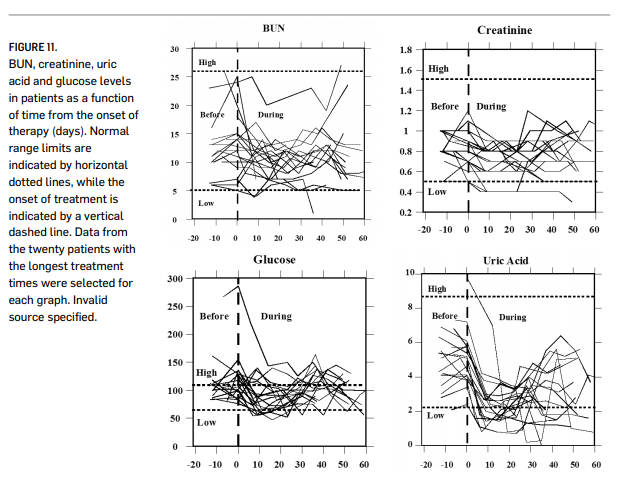
The safety of intravenous ascorbate has been addressed in recently published Phase I clinical studies (Riordan, et al., 2005; Hoffer, et al., 2008; Monti, et al., 2012). The first Phase I study was conducted with twenty-four terminal cancer patients (mostly liver and colorectal cancers) (Riordan, et al., 2005). The study used doses up to 710 mg/kg/day. Figure 11 (page 000) shows how parameters associated with renal function changed during the course of treatment. These indicators remained steady or decreased over time; this is significant since they would be expected to rise during treatment if ascorbate was having an acute detrimental effect on renal function. Blood chemistries suggested no compromise in renal function, and one patient showed stable disease, continuing treatment for an additional 48 weeks. Adverse effects reported were mostly minor (nausea, edema, dry mouth or skin). Two grade three adverse events “possibly related” to the agent were reported: a kidney stone in a patient with a history of renal calculus and a patient who experienced hypokalemia. These patients were generally vitamin C deficient at the start of treatment, and plasma ascorbate concentrations did not exceed 3.8 mM. In the study by Hoffer and coworkers (Hoffer, et al., 2008), twenty-four subjects with advanced cancer or hematologic malignancy not amenable to standard therapy were given IVC at doses of 0.4 g/kg to 1.5 g/kg (equivalent to a range of 28 to 125 grams in a 70 kg adult) three times weekly. In this study, peak plasma concentrations in excess of 10 mM were obtained, and no serious side effects were reported. Subjects at higher doses maintained physical quality of life, but no objective anticancer response was reported. The study by Monti and coworkers (Monti, et al., 2012), fourteen patients received IVC in addition to nucleoside analogue gemcitabine and the tyrosine-kinase inhibitor erlotinib. Observed adverse events were attributable to the chemotherapeutic agents, but not to the ascorbate, but no added efficacy due to the ascorbate was observed.
Thus far, Phase I studies indicate that IVC can be safely administered to terminal cancer patients at high doses (10 to 100 grams or more), but anticancer efficacy of the sort reported in case studies has not yet been observed. Of course, the terminal subjects used in Phase I studies would be expected to be the most difficult to treat. Phase II studies, with longer durations, are needed at this point.
Safety Issues Reported In Literature
Evidence indicates that patients who show no prior signs or history of renal malfunction are unlikely to suffer ill effects to their renal systems as a result of intravenous ascorbate (Riordan, et al., 2005). In cases where there are preexisting renal problems, however, caution is advised. In addition a kidney stone forming in one patient with a history of stone formation (Riordan, et al., 2005), a patient with bilateral urethral obstruction and renal insufficiency suffered acute oxalate neuropathy (Wong, et al., 1994). A full blood chemistry and urinalysis work-up is thus recommended prior to the onset of intravenous ascorbate therapy.
Campbell and Jack (Campbell & Jack, 1979) reported that one patient died due to massive tumor necrosis and hemorrhaging following an initial dose of intravenous ascorbate. It is thus recommended that treatment start at a low dose and be carried out using slow “drip” infusion. Fatal hemolysis can occur if a patient has glucose-6- phosphate dehydrogenase deficiency (G6PD). It is thus recommended that G6PD levels be assessed prior to the onset of therapy. The treatment is contraindicated in situations where increased fluids, sodium, or chelating may cause serious problems. These situations include congestive heart failure, edema, ascites, chronic hemodialysis, unusual iron overload, and inadequate hydration, or urine void volume (Rivers, 1987).
The Riordan IVC Protocol Inclusion Criteria and Candidates
1. Candidates include those who have failed standard treatment regimens; those seeking to improve the effectiveness of their standard cancer therapy; those seeking to decrease the severity and carcinogenicity of side effects from standard cancer therapy; those attempting to prolong their remission with health-enhancing strategies; those declining standard treatment, yet wishing to pursue primary, alternative treatment.
2. Patient (guardian or legally recognized caregiver) must sign a consent-to-treat or release form for the IVC treatment. Patient should have no significant psychiatric disorder, end-stage congestive heart failure (CHF), or other uncontrolled comorbid conditions.
3. Obtain baseline and screening laboratory:
a. Serum chemistry profile with electrolytes
b. Complete blood count (CBC) with differential
c. Red blood cell G6PD (must be normal)
d. Complete urinalysis
4. In order to properly assess the patient’s response to IVC therapy, obtain complete patient record information prior to beginning IVC therapy:
a. Tumor type and staging, including operative reports, pathology reports, special procedure reports, and other staging information. (Re-staging may be necessary if relapse and symptom progression has occurred since diagnosis.)
b. Appropriate tumor markers, CT, MRI, PET scans, bone scans, and x-ray imaging.
c. Prior cancer treatments, the patient’s response to each treatment type, including side effects.
d. The patient’s functional status with an Eastern Cooperative Oncology Group (ECOG) Performance Score.
e. Patient weight.
Precautions and Side Effects
In the Riordan Clinic’s experience giving over 40,000 onsite IVC treatments, the side effects of high-dose IVC are rare. However, there are precautions and potential side effects to consider.
1. The danger of diabetics on insulin incorrectly interpreting their glucometer finger stick has been found. It is important to notice to health care workers using this protocol for the treatment of cancer in patients who are also diabetic: high-dose intravenous vitamin C (IVC) at levels 15 grams and higher will cause a false positive on finger-stick blood glucose strips (electrochemical method) read on various glucometers (Jackson & Hunninghake, 2006). Depending on the dose, the false-positive glucose and occasionally “positive ketone” readings may last for eight hours after the infusion.
Blood taken from a vein and run in a laboratory using the hexokinase serum glucose method is not affected! The electrochemical strip cannot distinguish between ascorbic acid and glucose at high levels. Oral vitamin C does not have this effect. Please alert any diabetic patients of this potential complication! Diabetics wishing to know their blood sugar must have blood drawn from a vein and run in the laboratory using the hexokinase glucose determination method.
2. Tumor necrosis or tumor lysis syndrome has been reported in one patient after high-dose IVC (Campbell & Jack, 1979). For this reason, the protocol always begins with a small 15 gram dose.
3. Acute oxalate nephropathy (kidney stones) was reported in one patient with renal insufficiency who received a 60 gram IVC. Adequate renal function, hydration, and urine voiding capacity must be documented prior to starting high-dose IVC therapy. In our experience, however, the incidence of calcium oxalate stones during or following IVC is negligible (Riordan, et al., 2005).
4. Hemolysis has been reported in patients with G6PD deficiency when given high-dose IVC (Campbell, et al., 1975). The G6PD level should be assessed before beginning IVC. (At the Riordan Clinic, G6PD readings have yielded five cases of abnormally low levels. Subsequent IVC at 25 grams or less showed no hemolysis or adverse effects.)
5. IV-site irritation may occur at the infusion site when given in a vein and not a port. This can be caused by an infusion rate exceeding 1.0 gram/minute. The protocol suggests adding magnesium to reduce the incidence of vein irritation and spasm.
6. Due to the chelating effect of IVC, some patients may complain of shakiness due to low calcium or magnesium. An additional 1.0 mL of MgCl added to the IVC solution will usually resolve this. If severe, it can be treated with an IV push of 10 mLs of calcium gluconate, 1.0 mL per minute.
7. Eating before the IVC infusion is recommended to help reduce blood sugar fluctuations.
8. Given the amount of fluid used as a vehicle for the IVC, any condition that could be adversely affected by fluid or sodium overload (the IV ascorbate is buffered with sodium hydroxide and bicarbonate) is a relative contraindication (i.e. congestive heart failure, ascites, edema, etc).
9. There have been some reports of iron overload with vitamin C therapy. We have treated one patient with hemochromatosis with high-dose IVC with no adverse effects or significant changes in the iron status.
10. As with any IV infusion, infiltration at the site is possible. This is usually not a problem with ports. Our nursing staff has found that using #23 butterfly needles with a shallow insertion is very reliable with rare infiltrations (depending upon the status of the patient’s veins!).
11. IVC should only be given by slow intravenous drip at a rate of 0.5 grams per minute. (Rates up to 1.0 gram/minute are generally tolerable, but close observation is warranted. Patients
can develop nausea, shakes, and chills.)
12. It should never be given as an IV push, as the osmolality at high doses may cause sclerosing of peripheral veins, nor should it be given intramuscularly or subcutaneously. Table 1 lists the calculated osmolality of various amounts of fluid volume. Our experience has found that an osmolality of less than 1,200 milliosmole (mOsm)/kg H2O is tolerated by most patients. A low infusion rate (0.5 grams IVC per minute) also reduces the tonicity, although up to 1.0 grams per minute can be used in order to achieve higher post-IVC saturation levels. (Pre- and post-serum osmolality measurements are advisable at this dose.)
13. We presently use a sodium ascorbate solution, MEGA-C-PLUS®, 500 mg/mL, pH range 5.5–7.0 from Merit Pharmaceuticals, Los Angeles, CA, 90065.
Administration of IVC
Having taken all precautions listed above and having obtained informed consent from the patient, the administering physician begins with a series of three consecutive IVC infusions at the 15, 25, and 50 gram dosages followed by post-IVC plasma vitamin C levels in order to determine the oxidative burden for that patient so that subsequent IVCs can be optimally dosed.
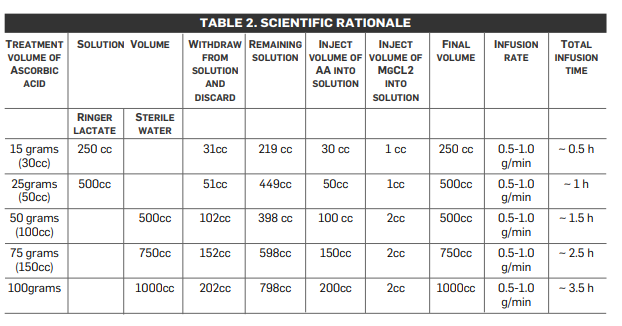
The initial three infusions are monitored with post-IVC infusion plasma vitamin C levels. As noted in Table 2 (below), research and experience has shown that a therapeutic goal of reaching a peak-plasma concentration of ~20 mM (350–400 mg/dL) is most efficacious. (No increased toxicity for post-IVC plasma vitamin C levels up to 780 mg/dL has been observed.) The first post-IVC plasma level following the 15-gram IVC has been shown to be clinically instructive: levels below 100 mg/dL correlate with higher levels of existent oxidative stress, presumably from higher tumor burden, chemo/radiation damage, hidden infection, or other oxidative insult, such as smoking.
Following the first three IVCs, the patient can be scheduled to continue either a 25- or 50-gram IVC dose (doctor’s discretion) twice a week until the post IVC plasma level results are available from the lab. If the initial 50-gram post-IVC level did not reach the therapeutic range of 350–400 mg/dL, another postIVC vitamin C level should be obtained after the next scheduled 50 gram IVC. If the therapeutic range is achieved, the patient is continued on a 50 gram twice a week IVC schedule with monthly post-IVC determinations to assure continued efficacy. If the therapeutic range is still not achieved, the IVC dosage is increased to 75 grams of vitamin C per infusion for four infusions, at which time a subsequent post-IVC plasma level is obtained. If the patient remains in a sub-therapeutic range, the IVC dosage is increased to the 100 gram level.
If after four infusions the post-IVC dosage remains sub-therapeutic, the patient may have an occult infection, may be secretly smoking, or may have tumor progression. While these possibilities are being addressed, the clinician can elect to increase the 100-gram IVC frequency to three times per week. Higher infusion doses beyond 100 grams are not recommended without serum osmolality testing before and after infusions in order to properly adjust the infusion rate to maintain a near physiologic osmolality range.
If higher dosages are not tolerated, or there is tumor progression in spite of achieving the therapeutic range, lower dosages can still augment the biological benefits of IVC, including enhanced immune response, reduction in pain, increased appetite, and a greater sense of well-being.
Very small patients, such as children, and very large obese patients need special dosing. Small patients [less than] 110 lbs. with small tumor burdens and without infection may only require 25 gram vitamin C infusions 2x/week to maintain therapeutic range. Large patients > 220 lbs. or patients with large tumor burdens or infection are more likely to require 100-grams IVC infusions 3x/week.
Post-IVC plasma levels serve as an excellent clinical guide to this special dosing.
In our experience, the majority of cancer patients require 50-gram IVC infusions 2–3x/week to maintain therapeutic IVC plasma levels. All patients reaching therapeutic range should still be monitored monthly with post-IVC plasma levels to ensure that these levels are maintained long term. We advise
patients to orally supplement with at least 4 grams of vitamin C daily, especially on the days when no infusions are given, to help prevent a possible vitamin C “rebound effect.” Oral alpha lipoic acid is also recommended on a case by case basis.
CONCLUSIONS
Vitamin C can be safely administered by intravenous infusion at maximum doses of 100 grams or less, provided the precautions outlined in this report are taken. At these doses, peak plasma ascorbate concentrations can exceed 20 mM.
There are several potential benefits to giving IVC to cancer patients that make it an ideal adjunctive care choice:
• Cancer patients are often depleted of vitamin C, and IVC provides an efficient means of restoring tissue stores.
• IVC has been shown to improve quality of life in cancer patients by a variety of metrics.
• IVC reduces inflammation (as measured by C-reactive protein levels) and reduces the production of pro-inflammatory cytokines.
• At high concentrations, ascorbate is preferentially toxic to tumor cells and is an angiogenesis inhibitor.
The next key step in researching the use of IVC for cancer would be Phase II studies, some of which are currently underway. IVC may also have a variety of other applications, such as combating infections, treating rheumatoid arthritis, and treating ADHD and other mental illnesses where inflammation may play a role.
REFERENCES
Agus, D., Vera, J. & Golde, D., 1999. Stromal cell oxidation: a mechanism by which tumors obtain vitamin C. Cancer Res., Volume 59, pp. 4555–8.
Ashino, H. et al., 2003. Novel function of ascorbic acid as an angiostatic factor. Angiogenesis, Volume 6, pp. 259–69.
Belin, S. et al., 2009. Antiproliferative effect of ascorbic acid is associated with inhibition of genes necessary to cell cycle progression. PLoS ONE, Volume 4, p. e4409.
Benade, L., Howard, T. & Burk, D., 1969. Synergistic killing of Ehrlich ascites carcinoma cells by ascorbate and 3-amino-1,2,4- triazole. Oncology, Volume 23, pp. 33–43.
Berlin, S. et al., 2009. Antiproliferative effect of ascorbic acid is associated with inhibition of genes necessary for cell cycle progression. PLoS ONE, Volume 4, pp. E44–0.
Block, K. et al., 2008. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Cancer, Volume 123, pp.
1227–39.
Cameron, E. & Pauling, L., 1976. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. PNAS USA, Volume 73, pp. 3685–9.
Cameron, E., Pauling, L. & Leibovitz, B., 1979. Ascorbic acid and cancer, a review. Cancer Res, Volume 39, pp. 663–81.
Campbell, A. & Jack, T., 1979. Acute reactions to mega ascorbic acid therapy in malignant disease. Scott Med J, Volume 24, p. 151.
Campbell, G., Steinberg, M. & Bower, J., 1975. Letter: ascorbic acid induced hemolysis in a G-6-PD deficiency.. Ann Intern Med, Volume 82, p. 810.
Casciari, J., Riordan, H., Miranda-Massari, J. & Gonzalez, M., 2005. Effects of high dose of ascorbate administration on L-10 tumor growth in guinea pigs. PRHSJ, Volume 24, pp. 145–50.
Casciari, J., Riordan, N. S. T. M. X., Jackson, J. & Riordan, H., 2001. Cytotoxicity of ascorbate, lipoic acid, and other antioxidants in hollow fibre in vitro tumours. Br. J. Cancer, Volume 84, pp. 1544–50.
Chen, Q. et al., 2008. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. PNAS USA, Volume 105, pp. 11105–9.
Chen, Q. et al., 2005. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. PNAS USA, Volume 205, pp.13604–13609.
Creagan, E. et al., 1979. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: A controlled trial. NEJM, Volume 301, pp. 687–690.
Drisko, J., Chapman, J. & Hunter, V., 2003. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. Am J Coll Nutr, Volume 22, pp. 118–23.
Du, J. et al., 2010. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res, Volume 16, pp. 509–20.
Espey, M. et al., 2011. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med, Volume 50, pp. 1610–19.
Espey, M., Chen, Q. & Levine, M., 2009. Comment re: vitamin C antagonizes the cytotoxic effects of chemotherapy. Cancer Research , Volume 69, p. 8830.
Frei, B. & Lawson, S., 2008. Vitamin C and cancer revisited.
PNAC USA, Volume 105, pp. 11037–8.
Fromberg, A. et al., 2011. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells toward cytostatic drugs.. Cancer Chemother Pharmacol, Volume 67, pp. 1157–66.
Fujita, K. et al., 1982. Reduction of adriamycin toxicity by ascorbate in mice and guinea pigs. Cancer Res, Volume 309–16, p. 42.
Geeraert, L., 2012. CAM-Cancer Consortium. Intravenous high dose vitamin C. [Online]
Available at: www.cam-cancer.ort/CAM-Summaries/Other-CAM/Intravenous-high-dose-vitamin-C.
Ginter, E., Bobeck, P. & Vargova, D., 1979. Tissue levels and optimal dosage of vitamin C in guinea pigs.. Nutr Metab, Volume 27,
pp. 217–26.
Gonzalez, M. et al., 2002. Inhibition of human breast cancer carcinoma cell proliferation by ascorbate and copper.. PRHSJ, Volume 21, pp. 21–3.
Heaney, M. et al., 2008. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res., Volume 68, pp.8031–8.
Henson, D., Block, G. & Levine, M., 1991. Ascorbic acid: biological functions and relation to cancer. JNCI, Volume 83, pp. 547–50.
Hoffer, L. et al., 208. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol, Volume 1969–74, p. 19.
Hoffman, F., 1985. Micronutrient requirements of cancer patients.. Cancer, 55(Supl. 1), pp. 145–50.
Hornig, D., 1975. Distribution of ascorbic acid metabolites and analogues in man and animals. Ann NY Acad Sci, Volume 258, pp. 103–18.
Jackson, J. & Hunninghake, R., 2006. False positive blood glucose readings after high-dose intravenous vitamin C. J Ortho Med, Volume 21, pp. 188–90.
Jackson, J., Riordan, H., Hunninghauke, R. & Riordan, N., 1995. High dose intravenous vitamin C and long time survival of a patient with cancer of the head and pancreas. J Ortho Med, Volume 10, pp. 87–8.
Keith, M. & Pelletier, O., 1974. Ascorbic acid concentrations in leukocytes and selected organs of guinea pigs in response to increasing ascorbic acid intake. Am J Clin Nutr, Volume 27, pp.368–72.
Kuether, C., Telford, I. & Roe, J., 1988. The relation of the blood level of ascorbic acid to tissue concentrations of this vitamin and the histology of the incisor teeth in the guinea pig. J Nutrition, Volume 28, pp. 347–58.
Kurbacher, C. et al., 1996. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett, Volume 103, pp. 183–9.
Levine, M. et al., 1996. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. PNAS USA, Volume 93, pp. 3704–9.
Lin, A., Chen, K., Chung, H. & Chang, S., 2010. The significance of plasma C-reactive protein in patients with elevated serum prostate specific antigen levels. Urological Sci, Volume 21, pp. 88–92.
Mayland, C., Bennett, M. & Allan, K., 2005. Vitamin C deficiency in cancer patients. Palliat Med, Volume 19, pp. 17–20.
McCormick, W., 1959. Cancer: a collagen disease, secondary to nutrition deficiency. Arch. Pediatr., Volume 76, pp. 166–171.
Mikirova, N., Casciari, J. & Riordan, N., 2012. Ascorbate inhibition of angiogenesis in aortic rings ex vivo and subcutaneous Matrigel plugs in vivo. J Angiogenesis Res, Volume 2, pp. 2–6.
Mikirova, N., Casciari, J., Taylor, P. & Rogers, A., 2012. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Trans Med, Volume 10, pp. 189–99.
Mikirova, N., Ichim, T. & Riordan, N., 2008. Anti-angiogenic effect of high doses of ascorbic acid.. J Transl Med, Volume 6, p. 50.
Mikirova, N., Rogers, A., Casciari, J. & Taylor, P., 2012. Effects of high dose intravenous ascorbic acid on the level of inflammation in patients with rheumatoid arthritis. Mod Res Inflamm, Volume 1, pp. 26–32.
Moertel, C. et al., 1985. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have no prior chemotherapy: a randomized double-blind comparison.. NEJM,
Volume 312, pp. 137–41.
Monti, D. et al., 2012. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One, Volume 7, p. e29794.
Murata, A., Morishige, F. & Yamaguchi, H., 1982. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Res Suppl, Volume 23, pp. 103–13.
Okunieff, P. & Suit, H., 1987. Toxicity, radiation sensitivity modification, and combined drug effects of ascorbic acid with misonidazole in vivo on FSaII murine firbo sarcomas. JNCI, Volume 79, pp. 377–81.
Padayatti, S. et al., 2006. Intravenous vitamin C as a cancer therapy: three cases. CMAJ, Volume 174, pp. 937–42.
Padayatty, S. & Levine, M., 2000. Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am Coll Nutr., Volume 19, pp. 423–5.
Padayatty, S. et al., 2010. Vitamin C: intravenous use by complementary and alternative medical practitioners and adverse effects. PLoS ONE, Volume 5, p. 11414.
Padayatty, S. et al., 2004. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med., Volume 140, pp. 533–37.
Page, E. et al., 2007. Hypoxia incudible factor-1 (alpha) stabilization in non-hypoxic conditions: role of oxidation and intracellular ascorbate depletion. Mol Biol Cell, Volume 19, pp. 86–94.
Pollard, H., Levine, M., Eidelman, O. & Pollard, M., 2010. Pharmacological ascorbic acid suppresses syngeneic tumor growth and metastases in hormone-refractory prostate cancer. In VIvo, Volume 2012, pp. 249–55.
Raloff, J., 2000. Antioxidants may help cancers thrive. ScienceNews, Volume 157, p. 5.
Riordan, H. et al., 2005. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. PR Health Sci J, Volume 24, pp. 269–76.
Riordan, H. et al., 2003. Intravenous ascorbic acid: protocol for its application and use. PR Health Sci. J., Volume 22, pp. 225–32.
Riordan, H., Jackson, J., Riordan, N. & Schultz, M., 1998. High dose intravenous vitamin C in the treatment of a patient with renal cell carcinoma of the kidney. J Ortho Med, Volume 13, pp. 72–3.
Riordan, N., JA, J. & Riordan, H., 1996. Intravenous vitamin C in a terminal cancer patient. J Ortho Med, Volume 11, pp. 80–2.
Riordan, N., Riordan, H. & Meng, X., 1995. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses, Volume 44, pp. 207–13.
Rivers, J., 1987. Safety of high-level vitamin C ingestion. In: Third Conference on Ascorbic Acid. Ann NY Acad Sci, Volume 489, pp. 95–102.
Shinozaki, K. et al., 2011. Ascorbic acid enhances radiation induced apoptosis in an HL60 human leukemia cell line. J Ratiat Res, Volume 52, pp. 229–37.
Simone, C., Simone, N. S. V. & CB, S., 2007. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase survival, part 1. Atlern Ther Health
Med, Volume 13, pp. 22–8.
St. Sauver, J. et al., 2009. Associations between C-reactive protein and benign prosaic hyperplasia lower urinary tract outcomes in a population based cohort. Am J Epidemiol, Volume 169, pp. 1281–90.
Taper, H., Keyeux, A. & Roberfroid, M., 1996. Potentiation of radiotherapy by nontoxic pretreatment with combined vitamins C and K3 in mice bearing solid transplantable tumor. Anticancer
Res, Volume 16, pp. 499–503.
Verrax, J. et al., 2004. Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic capsase-3 independent form of cell death. Apoptosis, Volume 9, pp. 223–33.
Verrax, J. & Calderon, P., 2009. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med, Volume 47, pp. 32–40.
Vollbracht, C. et al., 2011. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemoradiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo, Volume 82, pp. 983–90.
Wong, K. et al., 1994. Acute oxalate nephropathy after a massive intravenous dose of vitamin C. Aust ZN J Med, Volume 24, pp. 410–1.
Yeom, C., Jung, G. & Song, K., 2007. Changes of terminal cancer patients health related quality of life after high dose vitamin C administration. Korean Med Sci, Volume 22, pp. 7–11.
Yeom, C. et al., 2009. High-dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med, Volume 7, p. 70.