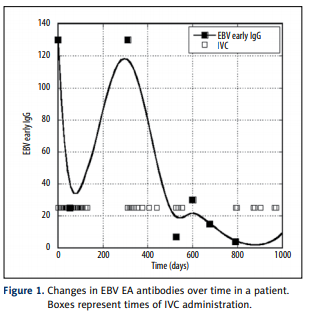
Effect of high dose vitamin C on Epstein-Barr viral infection
Effect of high dose vitamin C on Epstein-Barr
viral infection
Corresponding Author: Nina A. Mikirova, e-mail: nmikirova@riordanclinic.org
Source of support: Departmental sources
Background: Many natural compounds were tested for the ability to suppress viral replication. The present manuscript details an analysis of high dose vitamin C therapy on patients with EBV infection.
Material/Methods: The data were obtained from the patient history database at the Riordan Clinic. Among people in our database who were treated with intravenous vitamin C (7.5 g to 50 g infusions) between 1997 and 2006, 178 patients showed elevated levels of EBV EA IgG (range 25 to 211 AU) and 40 showed elevated levels of EBV VCA
IgM (range 25 to 140 AU). Most of these patients had a diagnosis of chronic fatigue syndrome, with the rest
being diagnosed as having mononucleosis, fatigue, or EBV infection.
Results: Our data provide evidence that high dose intravenous vitamin C therapy has a positive effect on disease duration and reduction of viral antibody levels.
Plasma levels of ascorbic acid and vitamin D were correlated with levels of antibodies to EBV. We found an
inverse correlation between EBV VCA IgM and vitamin C in plasma in patients with mononucleosis and CFS
meaning that patients with high levels of vitamin C tended to have lower levels of antigens in the acute state
of disease.
In addition, a relation was found between vitamin D levels and EBV EA IgG with lower levels of EBV early antigen IgG for higher levels of vitamin D.
Conclusions: The clinical study of ascorbic acid and EBV infection showed the reduction in EBV EA IgG and EBV VCA IgM antibody levels over time during IVC therapy that is consistent with observations from the literature that millimolar levels of ascorbate hinder viral infection and replication in vitro.
Background
The Epstein-Barr virus (EBV) is a member of the herpes family
that targets lymphocytes and epithelial cells [1–6]. It binds to
B-lymphocytes via the CD21 cell surface protein, and establishes
life-long persistence in memory B-cells [7–10]. While the infection is usually benign, it can in some cases lead to acute infectious mononucleosis and can impair the immune system [11,12].
EBV is linked to several malignancies, including Burkett’s lymphoma, post-transplant lymph-proliferative disease, Hodgkin’s
disease, and several autoimmune diseases [13–15]. EBV inhibits the ability of lymphocytes to respond properly to antigens
such as mitogenic lectins, concanavalin A, phytohemagglutinin, and pokeweed mitogen, among others [16,17].
EBV infections can be detected by immunoglobulin assays.
Most subjects show IgM antibodies to EBV viral capsid antigen (VCA) at the onset of infection, which decline after two to
six months. IgG antibodies to the EBV VCA may be detected
a few weeks or months after the onset of infection, and can
persist for life [9]. In addition, IgG antibodies to the EBV early
diffuse antigen (EA) can also be detected during acute infections [18,19]. Antibodies to the Epstein-Barr nuclear antigen
(EBNA) indicate the presence of a past infection. The profile
of antibodies that used to distinguish between the various
stages of EBV infection is summarized in www.cdc.gov. High
lymphocytes counts, particularly atypical high numbers of activated CD8 T-lymphocytes, and the presence of Downey cells
characterized by enlarged cytoplasm and condensed nuclei are
also present in primary EBV infection [20].
There is currently no treatment for removing EBV infections.
Our clinic has been long interested in the use of vitamin C
(ascorbic acid, ascorbate) to combat viral infections. Ascorbic
acid is an essential nutrient that functions as a key water soluble antioxidant and is involved in synthesis of collagen, carnitine, and neurotransmitters [21–23]. It affects wound healing, energy metabolism, nervous system function, and immune
cell health [24–27]. Oral supplementation with vitamin C typically gives rise to plasma ascorbate concentrations less than
0.2 mM, while high dose intravenous infusion of the vitamin
can raise plasma concentrations higher than 14 mM [28–30].
These “pharmacologic” plasma ascorbate concentrations
achieved by intravenous infusion have been linked with benefits to endothelial function, cellular immune function, antioxidative capacity, pain relief, and treatment of cancer and other illnesses [31–37].
The motivation for using intravenous infusions of vitamin
C (IVC) to treat viral illnesses comes, in part, from observations that virally infected patients exhibit vitamin C deficiency [38–40]. This in turn suggests that clinical management of
viral infections may benefit from supplementation. Improved
recovery of subjects with viral infection upon supplementation
with pharmacologic doses of vitamin C has been observed clinically [40–43]. In a multicenter cohort study, sixty-seven symptomatic Herpes-Zoster patients were given intravenous vitamin C in addition to standard treatment for shingles [43]. Pain
assessments were made and dermatologic symptoms such as
hemorrhagic lesions were followed during twelve weeks of
treatment. Pain scores, number of dermatomes and number
of efflorescences all showed statistically significant decreases during the treatment.
Several mechanisms of action have been proposed for this potential benefit. Since viral infections are often associated with oxidative stress, the ability of ascorbate replenishment to promote
a reducing environment could be important in detoxification and
neutralization of reactive oxygen species associated with infection [44]. Vitamin C is also necessary for neutrophil function, as
they typically accumulate ascorbic acid at eighty times the plasma concentration [45]. Also considered as potential mechanisms
are the ability of ascorbic acid to stimulate the production of interferon and other anti-viral cytokines, its ability to down regulate inflammation, and its direct antiviral properties [46–54].
The direct anti-viral activity of ascorbate has been studied extensively in vitro (animal studies are complicated by the fact
that most laboratory animals synthesize ascorbic acid). In one
study, for example, millimolar concentrations of ascorbate or
dehydroascorbate dramatically reduced the ability of three different viral types (herpes simplex virus type 1, influenza virus
type A, and picornaviridea virus 1) to infect cell monolayers
[51]. Note that millimolar concentrations of ascorbate are not
physiologically achievable through oral vitamin supplementation, but can be attained as a result of intravenous vitamin
C infusion. Suspensions of herpes simplex viruses (types 1
and 2), cytomegalovirus, and parainfluenza virus type 2 were
inactivated upon exposure to sodium ascorbate (at pharmacologic concentrations in the millimolar range) plus copper, and
ascorbic acid concentrations in the millimolar range were effective against human influenza viruses [42,52]. In chick embryo fibroblast, infection with Rous sarcoma viruses was inhibited by ascorbic acid [54].
The present manuscript details an analysis of EBV progression, via antibody assays, in patients undergoing intravenous
vitamin C therapy. Our results, detailed below, add further evidence to the idea that ascorbic acid may be useful in treating viral infections.
Material and Methods
The Epstein-Barr antibodies were detected in human serum
by enzyme linked immunosorbent assay (ELISA) according to manufacturer’s instructions (INCSTAR Corporation, Minnesota).
The absorbance of solution was measured at 450 nm. The normal range of antibodies to EBV viral capsid antigens IgG, IgM
and EBV early antigen IgG were in range 0–20 AU. PCR-based
method of EBV DNA detection in serum in conjunction with serological tests is a useful additional test to the panel of tests
offered at our clinical laboratory. However, test was not avalable and we did not have data for analysis.
The levels of vitamin C and vitamin D in blood were attained
by standard clinical procedures.
The study was conducted under Institutional Review Board
Approval of Riordan Clinic. Demographics were limited to ensure confidentiality, and informed consent was obtained from
all patients. From the database of patients with EBV infection
treated with IVC, we selected subjects for whom plasma antibody’ levels before and after treatment were available. The
details of the Riordan IVC protocol have been described elsewhere [29,30]. Briefly, patients are given IVC infusions at the
7.5, 15, 25, and 50 gram dosages. Dosages are adjusted by the
physician based on the patients’ tolerance and plasma ascorbic acid levels attained post infusion.
As hemolysis has been reported in patients with glucose6-phosphate dehydrogenase (G6PD) deficiency when given
high-dose IVC, the G6PD level was assessed for all patients
before beginning IVC.
The protocol also suggests adding magnesium to reduce the
incidence of vein irritation and spasm.
Statistical methods. The data were analysed by Systat software
(Systat, Inc) and Kaleidagraph software. Variables were presented as mean values ±SD, or as medians with corresponding 25th percentiles. Association between different factors
was assessed using linear models. Statistical significance was
accepted if the null hypothesis could be rejected at p£0.05.
Results
The data were obtained from the patient history database at the
Riordan Clinic, a nutritional medicine treatment and research
clinic. Among people in our database who were treated at the
clinic with intravenous vitamin C (7.5 g to 50 g infusions) for
various illness, we found 178 patients who showed elevated
levels of EBV IgG (range 25 to 211 AU) and forty who showed
elevated levels of EBV VCA IgM (range 25 to 140 AU). These
subjects, all being treated between 1997 and 2006, formed the
basis of our study. Most of these patients (110 subjects) had
a diagnosis of chronic fatigue syndrome, with the rest being
diagnosed as having mononucleosis, fatigue, or EBV infection.