Continuous Intravenous Vitamin C in the Cancer Treatment: Reevaluation of a Phase I Clinical Study
Authors: Nina Mikirova, PhD, Joseph Casciari, PhD, Ronald Hunninghake, MD
Published: Functional Foods in Health and Disease, March 31, 2019
ABSTRACT
Background: Intravenous high-dose vitamin C (IVC) therapy is widely used in naturopathic and integrative oncology. A number of Phase I and Phase II clinical trials were launched to prove the benefits of the IVC therapy. Many case studies demonstrated the effectiveness of IVC, with various degrees of success. Clinical trials using IVC to treat cancer have, to date, demonstrated
its safety without conclusively proven its efficacy. One difficulty in administering IVC is determining the optimal treatment schedule. To this end, data from a previous Phase 1 clinical
trial conducted in 1998 using continuous vitamin C infusions was analyzed to examine the effects of this regimen on key prognostic parameters.
Method: Twenty-four subjects were given continuous IVC at doses between 150 and 710 mg/kg/day. Most of the patients had colon cancer with liver and lung metastasis and three patients had pancreatic or liver cancer. All patients had several chemotherapy/radiation treatments before entering the study. Patients were treated by pharmaceutical grade sodium ascorbate diluted in lactated Ringers solution with the rate of infusion of 20 ml/hr or 10 ml/hr for lower doses. This diluted solution was administered by continuous infusion.
Results: Prior to treatment, serum lymphocyte counts and ascorbate concentrations tended to be low while serum levels of lactate dehydrogenase (LDH), neutrophils, and glucose tended to be high. Improvements were seen during IVC therapy. In patients with initially elevated neutrophil levels, numbers tended to decrease. In contrast, increased absolute neutrophil and lymphocyte numbers were seen in patients with initially low counts. Neutrophil to lymphocyte ratios (NLR) proved to be a good indicator of cancer patients’ survival times (high NLR, low survival). This was also true of LDH, creatinine, and glucose concentrations. In patients with the highest pretreatment NLR, rate of growth of this ratio decreased significantly during therapy. IVC treatments were also associated with decreases in glucose concentrations, restoration of vitamin C levels, and, in about 40% of cases, reductions in LDH levels.
Conclusions: As the result of the study we found that continuous IVC infusions improved several parameters associated with poor cancer prognosis. The data suggests a strategic benefit to using lower IVC doses in continuous infusions: raising the dose above 300 mg/kg/day (20 grams in 70 kg human) increased the frequency of side effects without noticeably increasing plasma ascorbate levels. Moreover, improvements in lymphocyte counts at low IVC doses tended to decrease at the higher doses. In conclusion, continuous infusions had benefits to cancer patients and further research in this area is warranted.
Keywords: ascorbic acid; continuous infusion; cancer patients; clinical trial; lymphopenia; neutrophil to lymphocyte ratio; hyperglycemia; safety.
INTRODUCTION
Intravenous vitamin C therapy has garnered increased interest as a potential treatment for cancer [1-3] and is being used widely in naturopathic and integrative oncology [4]. IVC was first proposed for cancer treatment in the 1970’s [5].
Preclinical studies of large doses of ascorbic acid (vitamin C) have been reported to show significant anti-cancer effects in animal models and tissue culture investigations [6-12]. These include direct cytotoxic effects in certain cancer cell lines at micromolar (μM) to millimolar (mM) concentrations. The data indicates that pharmacologic ascorbate concentrations, where ascorbate is present in sufficient quantities, acts as a drug [13-17].
Studies on understanding the biological activities of ascorbate have led to a number of hypotheses for mechanisms of anti-cancer activity, such as the generation of significant quantities of hydrogen peroxide by the autoxidation of pharmacological concentrations of ascorbate [7, 8], changes in the metabolic activity [18], and stimulation of the 2-oxoglutarate dependent dioxygenase family of enzymes (2-OGDDs) that have a cofactor requirement for ascorbate [14, 17]. The 2-OGDDs include the hydroxylases that regulate the hypoxic response, a major driver of tumor survival, angiogenesis, and metastasis, and the epigenetic histone and DNA demethylases [13, 14, 17].
High dose ascorbic acid may improve the anti-cancer action of chemotherapeutic agents [19- 21], boost immune cell functioning [22], and inhibit angiogenesis [23]. Pharmacokinetic studies
indicate that high ascorbate concentrations associated with these phenomena may be achievable in vivo, provided ascorbate is administered intravenously [24-26]. Many case studies demonstrated the effectiveness of intravenous vitamin C, with various degrees of success [27-33]. Clinically published IVC case studies report efficacy against a variety of cancers in humans,
including pancreatic cancer, bone metastases accompanying breast cancer, non-Hodgkin’s lymphoma, liver carcinoma, colon carcinoma, and ovarian cancer.
However, high dose IVC treatment has not yet been proven to be a cancer cure. As part of a comprehensive cancer treatment program, vitamin C was proven to be a powerful tool to help people to have stronger immune systems and an improvement in the quality of life. IVC treatment reduces levels of inflammation markers, ameliorates symptoms in cancer patients, and increases chances of surviving their disease [34-37].
Several Phase I and Phase II clinical trials have been conducted in the last ten years to test safety and efficacy when IVC is used as an adjuvant with chemotherapy [38-42]. The results of these trials confirm that IVC can be administered safely. However, there are mixed results concerning efficacy.
Thus, many researchers consider the greatest potential for IVC to be as an adjuvant to conventional treatment. Some support for this notion includes the observation that cancer patients tend to be deficient in vitamin C. This ascorbate deficiency correlates with inflammation, infection, and disease. Moreover, it is exacerbated by chemotherapy and radiation [43-47].
The effectiveness of chemotherapeutic regimens is often dependent upon the treatment schedule used, with large intermittent doses often being more toxic and less effective than smaller repeated doses [48]. The response to treatment may not be proportional to cumulative drug dose or the area under the disposition curve [49-51]. Instead, the time during which the drug concentration is maintained close to target concentration may be a more important factor for antitumor activity and highest efficacy of treatment [48].
In most clinical applications, IVC is administered through a one-hour infusion two or three times a week. In a Phase I clinical trial conducted by Riordan et al., however, patients were given continuous infusions using an infusion pump [37]. This may represent a more favorable treatment schedule. Therefore, we decided to study this trial in more detail.
The published report on this Phase I trial focused on plasma ascorbate levels attained, effect on blood chemistry parameters associated with renal function, and adverse events [37]. The
report concluded that all doses tested were safe provided the subject did not have a history of kidney stones.
In the present manuscript, previously unpublished parameters from the Riordan clinical study, including blood chemistry and blood count parameters that are reportedly related to patient prognosis and degree of inflammation are examined further. This includes absolute neutrophil and lymphocyte counts and the neutrophil-to-lymphocyte ratio (NLR). NLR in particular has been shown to be useful in predicting survival in cancer patients [52-54]. Blood chemistry parameters analyzed include: lactate dehydrogenase, an enzyme involved in tumor initiation, metastasis, and recurrence that serves as a prognostic marker for poor cancer outcome [55-57]; creatinine, the depletion of which is associated with cachexia [58-60]; and glucose, as hyperglycemia is common in cancer patients and there is evidence that vitamin C reduces hyperglycemia [61, 62]. We report that IVC had positive effects on several of these parameters during the Phase I study of continuous IVC infusion that we analyzed.
Moreover, our analysis suggests that the higher doses within the study were associated with more side effects, but were not associated with dramatically increasing benefits.
MATERIALS AND METHODS
A detailed description of how the Phase 1 IVC continuous infusion clinical trial was conducted was given previously [37]. Briefly, patients were divided into groups and treated by continuous
infusion. A total of 24 patients with late-stage cancer were included in our study. The characteristics of each patient, along with the ascorbate dose each patient was given, and prior therapies are listed in Table 1.
Table 1. Characteristics of twenty-four cancer patients participating in a phase I clinical trial of continuous IVC infusions.
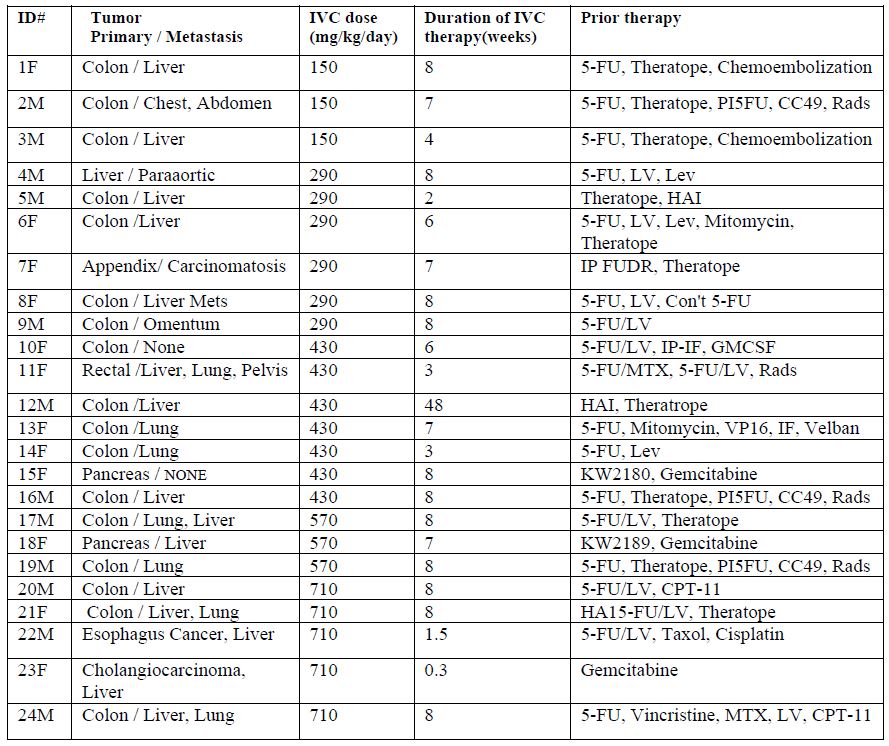
Fifty percent of the patients were males and fifty percent were females (in the ID# in Table 1, “M” refers to male and “F” refers to female). All patients had several rounds of chemotherapy or radiation prior to entering the study. 79% of the patients had a metastatic tumor. 71% (17patients) had colon cancer with liver and lung metastasis, three patients had pancreatic or liver cancer and the rest of the patients had esophagus or rectal cancer.
The investigation was carried out following the rules of the Declaration of Helsinki of 1975 (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/), revised in 2008. Written informed consent was provided by all patients. The study was approved by the ethics committee of the Eppley Institute for Research in Cancer and Allied Diseases at the University of Nebraska Medical Center (Omaha, NE) and the Institutional Review Board of the Riordan Clinic (Wichita, KS).
Patients were divided into five groups and treated by continuous infusion of 150 mg/kg/day (three patients), 290 mg/kg/day (seven patients), 430 mg/kg/day (six patients), 510 mg/kg/day (three patients) and 710 mg/kg/day (five patients). Pharmaceutical grade sodium ascorbate was diluted in Lactated Ringers solution and infused with the rate of infusion of 20 mL/hr or 10 ml/hr for lower doses. The diluted solution was administered by continuous infusion using a Travenol Infuser (Pharmacia Deltac, St. Paul, MN) with a Cad-5400 or Sabrateck 6060 infusion pump. The ascorbate solution was changed daily. The infusion system was flushed with 100 mL normal saline daily to prevent buildup of crystals in the access line and then “reloaded” with fresh ascorbate. The duration of the continuous infusion was at least 20-22 hours.
Patients’ health, adverse events, and tumor progression were monitored during treatment. Samples for routine blood chemistry were collected one week prior to therapy and at roughly weekly intervals during treatment. White blood cell counts, hemoglobin, hematocrit, red blood cell counts, and standard blood chemistry parameters were determined by using standard procedures at the Eppley Institute for Research in Cancer at the University of Nebraska Medical Center (Omaha, NE). Plasma vitamin C concentrations were measured as a function of time for twenty-two of the twenty-four patients. Serum was collected for this purpose one week prior to therapy, daily for the first four days of therapy and weekly thereafter. To determine ascorbate concentrations plasma samples were stabilized in 3% metaphosphoric acid and sent to the Bio-Center Laboratory (Wichita, KS) for colorimetric analysis by the reduction of 2,6-dichlorophenolindophenol. The lower limit of ascorbate detection was 0.2 mg/dL.
The data were analyzed by Systat software (Systat, Inc) and Kaleidagraph software. Variables were presented as means ± SD, or as medians with corresponding 25th and 75th percentiles. Association between different conditions and factors were assessed by using nonparametric statistics (Spearmen’s rank correlation coefficient and t-value).
RESULTS
General pre-treatment parameters and patient outcome
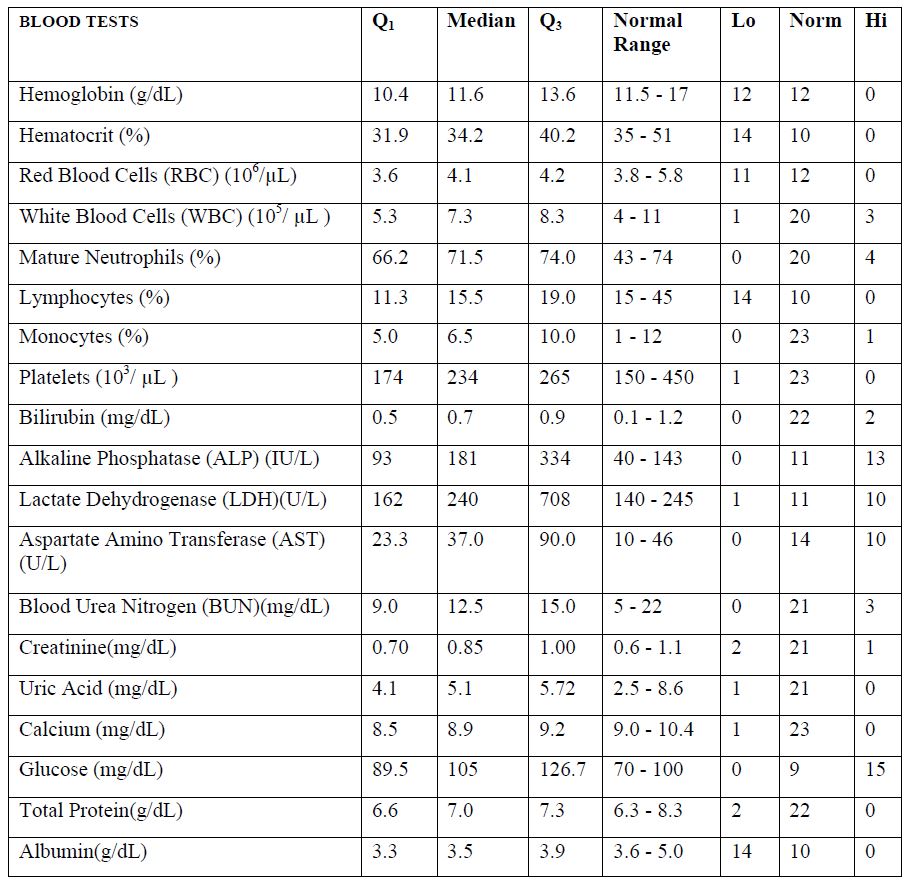
Median values of blood test results for patients prior to IVC therapy are presented in Table 2, along with first and third quartiles and a breakdown of how many values fell above, below, or
within the normal range for each parameter.
According to these data, blood levels for hemoglobin, hematocrit, RBC, lymphocytes, and albumin tended to be lower than the levels of normal range, while the medians for ALP, LDH, AST, and glucose tended to be higher.
The most common outcome was progressive disease, defined as a twenty-five percent or greater increase in size of all measurable lesions. This was expected for a patient cohort of this sort. However, one patient had stable disease (no increase in size of pre-existing lesions and no new lesions). Each patient was treated over an eight-week period, unless an adverse event or progression of disease required that the treatment be stopped. Among those with progressive disease, eleven completed the eight weeks of therapy. Two were removed from the study due to Grade Three of Four adverse events and two elected to stop treatment due to problems with venous catheter occlusion. The remainders were removed from the trial before the eight-week time period expired due to progressive disease. Overall, eighteen of the patients received the treatment for at least six weeks. One subject, a man with colon cancer and liver metastasis who was treated with 430 mg/kg/day ascorbate showed stable symptoms and test results and elected to continue ascorbate therapy for an additional forty-eight weeks. He survived a total of 336 days from the onset of therapy. The average survival of the patients from the beginning of the treatment was 110 days (IQR = 63 – 304 days).
Table 2. Blood test results for participants in Phase 1 study prior to IVC therapy. Q1 and Q3 refer to the first and third quartile values, respectively. “Lo” and “Hi” refer to the number of values below and above normal range, respectively.
Plasma ascorbate levels
The concentration of ascorbic acid in blood was measured the first four days and at the end of each week of treatment. The levels of ascorbic acid that were achieved in the blood are shown in
Figure 1.
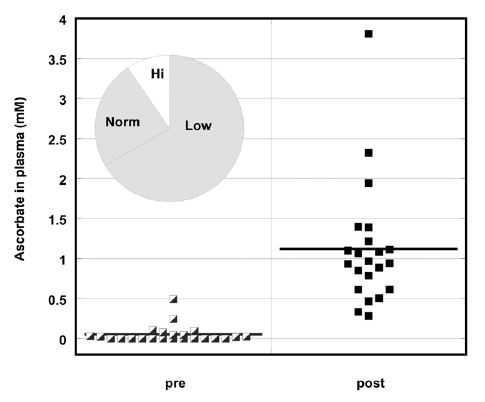
Figure 1. Ascorbate concentration (mM) in plasma before and during IVC treatment. Pie chart insert shows number of patients with low (Lo), high (Hi), or normal (Norm) ascorbate levels
before treatment.
Pre-values are the individual measurements of ascorbic acid for each subject before treatment and post-values are the average ascorbic acid concentrations in blood for each patient during treatment. The pie chart insert refers to the number of subjects who showed normal (34%) or below normal (66%) plasma vitamin C concentrations. Two thirds of the subjects had levels below the normal range (0.6mg/dL-2mg/dL), with the majority of them (47%) having levels undetectable by colorimetric assay. We should note that at this time the clinical laboratory used a colorimetric method for measurements of ascorbate, which was not very sensitive. During IVC treatment, ascorbate levels increased to a mean value (for all patients) of 1.1 mM.
Figure 2 shows the examples of the plasma concentrations versus time graphs for several patients given continuous infusions of 150 mg/kg/day to 710 mg/kg/day IVC.
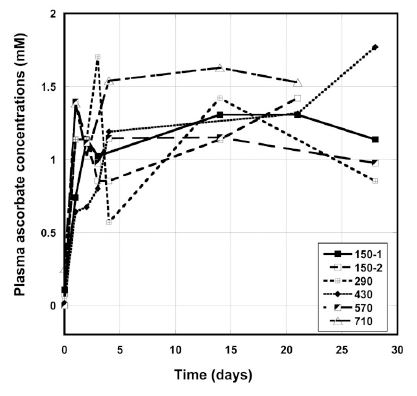
Figure 2. Plasma ascorbate concentrations (mM) during IVC therapy for several patients given a dose 150 mg/kg/day – 710 mg/kg/day by continuous infusion.
The plasma concentration rose in the first few days, and then remained in the range 0.8 mM -1.7 mM. We did not see dependence of the average plasma ascorbate concentrations after IVC
treatment as a function of dosage. The mean plasma ascorbate level, at steady state, for the three subjects at the dose 150 mg/kg/day was 1.00 ± 0.34 (SD) mM. Higher doses did not significantly
increase plasma ascorbate values, and at higher dosages the average concentration during the IVC treatment reached 1.3 mM ± 1.4 mM for 290 mg/kg/day, 0.9 mM ± 0.6 mM for 430 mg/kg/day, 1.0 mM ± 0.5 mM for 570 mg/kg/day and 1.4 mM ± 0.8 for 710 mg/kg/day. For four patients the level of ascorbate in blood reached much higher concentrations (2.8 mM to 5 mM), that probably, can be explained by decreased clearance of ascorbate by kidneys. One of these patients had rectal cancer and three others liver and colon cancers with metastasis.
The leveling off of the ascorbate concentration is consistent with observations that high doses will saturate renal tubular ascorbate reabsorption, leading to increased ascorbate excretion [63]. Our data suggest that, when IVC is provided by continuous infusion, plasma concentrations quickly reach ~ 1 mM and are maintained, but that increasing dosage did not provide added benefit.
Neutrophil and lymphocyte counts
As lymphocytes and neutrophils have important roles in tumorigenesis and carcinogenesis, we analyzed the effect of the treatment on these parameters. In chemotherapy, neutrophil and lymphocyte counts typically decrease, with the effect being more severe for lymphocytes [64,65]. For cancer patients in general, increased neutrophil counts are consistent with systemic inflammation. The normal range for absolute neutrophil counts (ANC) is 2000 to 7000 cells/μL, while that for absolute lymphocyte counts (ALC) is 1300 to 4000 cells/μL. Patients in the study
did not have neutropenia, nor did they develop it during treatment, but more than half of the patients had lymphopenia.
Mean value of ANC for all patients prior to treatment was 5240 ± 1850 (SD) cells/μL and 5630 ± 1740 (SD) cells/μL after therapy. Prior to therapy, neutrophil counts were elevated in three subjects. Subjects with low ANC and ALC values tended to see those values increase. We noticed, however, that such increases were not prevalent for patients who initially had ANC or ALC values in normal range. We tested this by plotting the changes in ANC or ALC values versus the initial values (Figures 3 A, B).
To find the effect of IVC treatment on neutrophil count, we analyzed the changes in the average absolute neutrophil counts before and after treatment.
The percentage of change of ANC for patients who completed 6-8 weeks of treatment is shown in Figure 3 (A). These values were higher than normal range for two patients with metastatic colon cancer (8250 cells/μl and 9417cell/μl) and decreased after treatment on 10% ÷ 37%. Another patient with metastatic colon cancer had ANC at the upper level of normal range and his value was increased on 38%, demonstrating the increased level of inflammation in this patient. Two patients with pancreatic cancer with metastasis had the initial levels of ANC on the lower level of normal range (2570 cells/μl and 2930 cells/μl), which increased to 46% and 94% at the end of the treatment. For the rest of the patients the tendency was in improving the ANC at the low level of this parameter and decreasing for the higher values.
In addition, we investigated the effect of treatment on the absolute lymphocyte count. Mean value of ALC for all patients prior to treatment was 1570 ± 1590 (SD) cells/μL. The initial level of ALC was lower than normal range (1300 /μl – 4000 /μl) for 14 of 24 patients who started intervention.
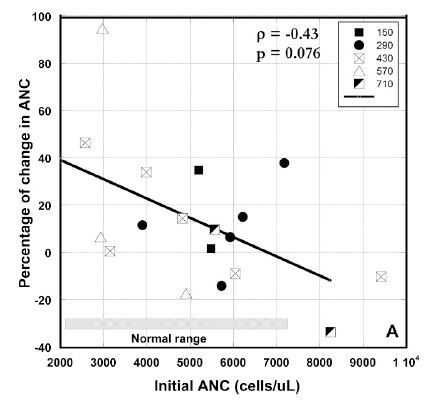
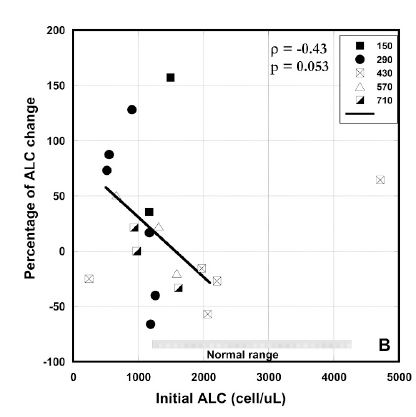
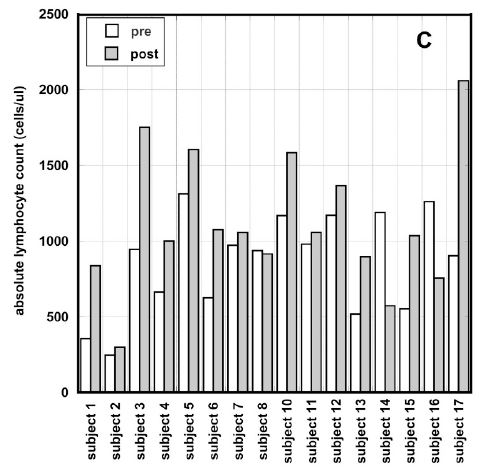
Figure 3 (A, B, C). Percentage of change in absolute neutrophil counts (A), percentage of change absolute lymphocyte count vs. pre-treatment levels (B) and absolute values of ALCs before and after intervention for patients with ALCs lower than normal range (C). Different dosages in Figures 3(A, B) are indicated by the different shapes. Spearmen’s rank correlation coefficient, non-parametric p-values, and regression lines are given.
Severe lymphopenia (ALC<1000 cells/μl) was measured in 10 patients. Only six patients with severe lymphopenia completed 6-8 weeks of treatment. The most severe lymphopenia was measured in a patient with pancreatic cancer (245 cells/μl) and in a patient with rectal cancer/liver, lung, pelvis metastasis (325/μl). The percentage of improvement in ALC was calculated based on the initial ALC values and the ALC at the end of the treatment. In average, for six patients with the ALC <1000 cells/μl there was improvement in the lymphocyte count at the end of the treatment on 69% (median), IQR: 129%, -6%.
The highest improvement was seen in a female with rectal cancer liver, lung and pelvis metastasis (355 cells/μl initial and 135% improvement). Two patients with metastatic colon cancer and ALC counts 520/μl and 900/μl had improvement in ALC of 73% and 128%. Another patient, the female subject with pancreatic cancer/liver metastasis and initial ALC 663/μL had improvement in the score of 50%. In the patient with carcinomatosis, with initial ALC of 550/μL, the increase in lymphocyte count was 87%. There was no improvement for the patient with pancreatic cancer who had the lowest initial ALC score of 245/μL.
In average the improvement in lymphocyte count for all patients who completed 6-8 weeks of treatment and had ALC <1300 /ul was 22% (IQR: 89%, -24%). Results are shown in Figure 3(B). According to these data there was improvement in ALC for patients with ALC below the normal range. In addition, the absolute counts of lymphocytes pre and post intervention for patients with low initial ALCs are shown in Figure 3 (C). According to these data there was improvement in ALC for patients with ALC below the normal range. For five patients the ALC values returned to the normal level (ALC>1300 cells/ul) and for five patients the values reached the level 1000 cells/ul.
As we had values of ALC one week upon study entry, at the beginning of the study and at the end of every week, the trend in this parameter before and during treatment was evaluated. The data demonstrates that before treatment there was a decrease in ALC with median -9.8% (IQR:-20%, 13.5%), but after treatment the median increase in ALC was 22% (IQR: -8.9%, 73%).
These data show that continuous IVC can have a positive effect on the improvement of lymphocyte count and immune function in patients with lymphopenia.
There was a correlation between initial values and percentage of change in ANC and ALC where increasing initial value corresponded to decreasing final values seen in each case (Spearman’s ρ values of –0.43 for each), yielding, for two tailed testing, p-values between 0.10 and 0.05. This suggests a potentially useful moderation of ANC and ALC where levels that are decreased due to a factor such as chemotherapy are restored, while levels that are increased due to inflammation are reduced.
We analyzed to see if there was improvement of the absolute lymphocyte count with increasing dosages of infusion. For all patients who completed 6-8 weeks of treatment, the effect of vitamin C dosage on the change in lymphocyte counts was examined.
At the low doses (150 or 290 mg/kg/day IVC), the median change in lymphocyte counts was 9%, with four subjects seeing increases and three seeing decreases. With the high doses (430, 570, and 710 mg/kg/day), however, the median change in lymphocyte counts was – 12 %, with seven subjects seeing decreases and only two seeing increases (with two patients showing virtually no change in ΔALC). These data may indicate that lower doses are more favorable for the improvement of lymphocyte count.
The neutrophil-to-lymphocyte ratio may be a useful prognostic factor in a variety of cancers [53], with higher values indicating lower survival times. We examined the rate of change in this ratio (ΔNLR) for each patient before and after therapy. To calculate the initial ΔNLR (prior to therapy), NLR on day zero was subtracted from NLR measured one week prior to therapy, and this difference was divided by the number of days between the two measurements. Similarly, the final ΔNLR was calculated based on the last two measurements during therapy. Changes in these values for thirteen patients who completed 6-8 weeks of treatment and for whom pre-treatment data were available are shown in Figure 4 (A).
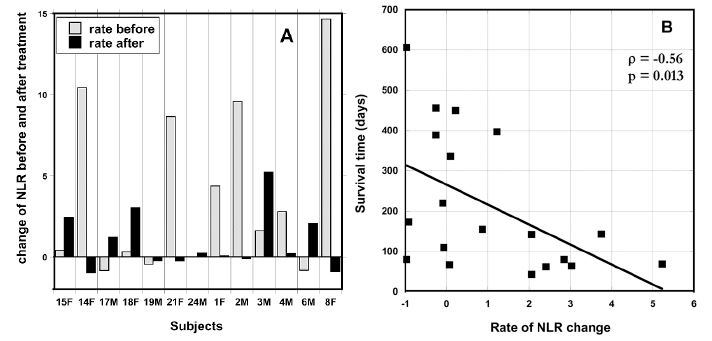
Figure 4. (A) ΔNLR values pre-IVC and at the end IVC therapy (post). (B) Correlation between patient survival time (days after start of therapy) and the ΔNLR value at the end of therapy.
At the beginning of IVC therapy 75 % of subjects had increasing NLR levels higher than normal range (0.78 – 3.53) and at the end of IVC therapy the same percentage of patients still had increased levels of NRL, but the comparison of the trend in the change of NLR measured for periods one week before treatment and during treatment demonstrated that the rate of change was decreased. The median ΔNLR values were 2.19 pre-therapy and 0.21 post-therapy (p = 0.045).
Data in Figure 4 (A) show the effect of the treatment on the NLR rate of change. The crossed bars in the graph present the rate of increase in NLR before treatment and black bars show the NLR growth after treatment. The six subjects who had the highest pre-therapy increase in NRL showed a decrease in ΔNRL during therapy. According to these data, the treatment resulted in the suppression or prevention of the progression of the rate of growth of NLR. This improvement of the rate of change of NLR was found for 54% of the patients. For patients with initial NLR higher than upper level of normal level of 3.53, the improvement was seen in 64% of patients who completed 6-8 weeks of treatment.
Our data also demonstrates the relationship between the survival of patients and the rate of growth of NLR. Figure 4(B) shows a statistically significant (p = 0.013) correlation between post-treatment ΔNLR and survival time, with high ΔNRL coinciding with lower survival times. This suggests that IVC may reduce NLR levels, thus improving prognosis.
Blood chemistry parameters
The normal range for blood lactate dehydrogenase is between 140 U/L and 280 U/L. LDH concentrations before IVC therapy were above the normal range in 50% of the patients (LDH range 300 U/L – 1790 U/L). The median LDH prior to therapy was 240 U/L (IQR: 627 U/L, 161 U/L) while that after IVC therapy was 276 U/L (IQR: 739 U/L, 156 U/L).
The rate of increase of LDH was calculated before and after treatment. The value of this parameter (LDH rate of growth) was decreased in 38% of the patients, increased in 28.6% and was not changed in 33.4% of patients. The result that LDH decreased in 38% of the subjects is remarkable considering their illness.
The survival of patients was compared to those with normal and higher than normal range (NR) initial blood LDH levels in Figure 5.
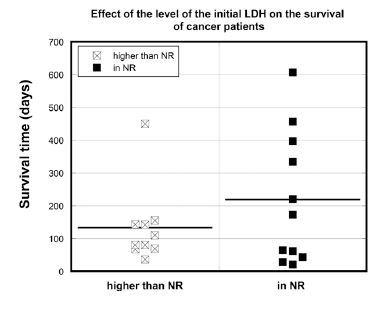
Figure 5. Survival times (days) of cancer patients with normal (LDH<245 U/L) or above normal range (NR) (LDH>245 U/L) LDH concentrations.
The median survival time for the all participants with initial LDH higher than normal range (LDH>245 U/L) was 95 days. In contrast, the median survival time for all subjects with normal initial LDH values was 173 days (p = 0.097). The median survival time for the patients who were able to complete the 6-8 weeks of the study was 153 days for patients with initial LDH higher than normal range and 238 days for patients with initial LDH within the normal range. As activation of glycolytic metabolism is a significant characteristic of tumor cells, and since lactate dehydrogenase is an important coenzyme in glycolysis, elevated levels of serum LDH may be useful prognostic biomarkers [55, 56].
Blood creatinine levels were also examined. Before intervention the level of creatinine was in normal range (0.5-1.0 mg/dl for women and 0.7-1.2 mg/dl for men) for 23 patients, the exception being a female with pancreatic cancer. During treatment the level of creatinine was decreased (median -14%, IQR: -5.5%, -23.5%) and for seven patients fell below the normal range.
Creatinine is a metabolite of L-carnitine, which plays a central role in the metabolism of fatty acids. Low serum creatinine and carnitine levels are associated with the muscle wasting seen in cancer patients, which is particularly severe in patients with colon and pancreatic cancers [59, 60]. These cancers were prominent in this study (Table 1).
We calculated the percentage of change of the average creatinine concentration before treatment and during treatment, and divided all patients in two groups with higher and lower than 20% change in creatinine levels. The median survival time for patients who had a decrease of creatinine greater than 20% was 80 days (IQR: 142, 62) and 254 days (IQR: 451, 123) for patients with a creatinine change less than 20%. Large decreases in creatinine concentration correlated with low survival times (p = 0.043), as shown in Figure 6, consistent with prior results [60].
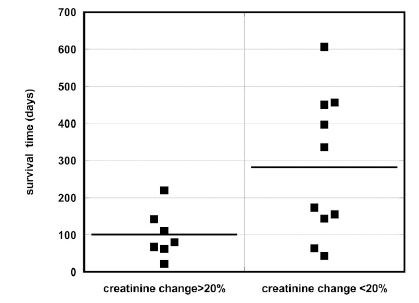
Figure 6. Survival time for patients with a greater than 20% decrease in creatinine levels versus those with a less than 20% decrease.
Hyperglycemia is common in cancer patients. Two-thirds of the patients in our study had above normal blood glucose concentrations (Table 2). Changes in blood glucose during IVC therapy for patients with the highest blood glucose levels are shown in Figure 7(A). For these patients, glucose concentrations decreased during IVC therapy.
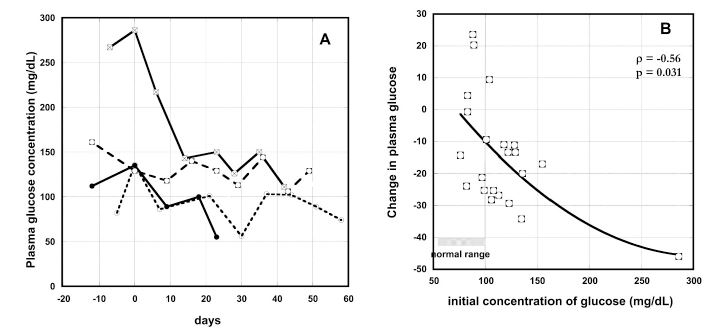
Figure 7. (A) Change in plasma glucose concentrations (mg/dL) with time of IVC therapy for several cancer patients (time zero represent the onset of therapy). (B) Percentage of plasma glucose concentration change during IVC therapy as function of pre-treatment glucose concentrations (mg/dL). Spearman’s rank correlation coefficient and the p-value for significance are given. The line represents the second order polynomial extrapolation of the data.
For all patients with increased level of glucose the concentration of glucose was decreased on – 11% ÷ -45% during treatment. Figure 7(b) shows the correlation between initial glucose concentration and the change in glucose levels during IVC therapy. Glucose levels decreased during therapy most dramatically when the initial glucose concentrations were higher.
Safety and side effects
Side effects and safety were discussed in detail in the previous article [37]. Briefly, blood chemistry parameters that serve as indicators of renal function (BUN, creatinine, and uric acid) remained relatively stable or in the case of uric acid, decreased during therapy. Only four subjects experienced BUN increases during therapy.
Adverse events were assessed by the physician using the NCI Common Toxicity Criterion and were attributed to the agent according to the grades “not related”, “possibly related”, or “probably related”. The adverse effects that were experienced by patients are presented in Figure 8. The most common side effects were nausea (11 subjects), injector port occlusion (10 subjects), dry skin or mouth (7 subjects), edema (7 subjects) and fatigue (6 subjects). These were generally minor (Grade 1).
A total of five Grade 3 adverse events were observed during the study, with only four of them being considered possibly related to the therapy (the only Grade 4 event observed was cardiac arrest, which was considered unrelated to the therapy). One patient developed a kidney stone after thirteen days of treatment at 290 mg/kg/day ascorbate. Acute renal distress is usually accompanied by an increase in glucose, creatinine, urea and BUN levels; however, this subject’s blood creatinine, glucose and BUN levels remained stable, but this subject had a prior history of kidney stones [37].
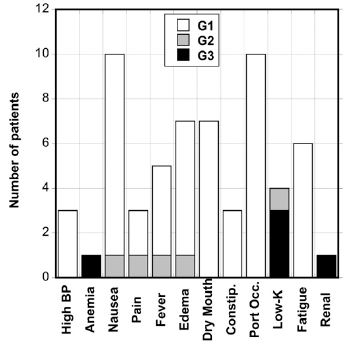
Figure 8. Grade 1, 2, and 3 adverse events reported for patients given IVC infusion therapy.
Most of the Grade 3 events involved hypokalemia, which is considered possibly related to the ascorbate therapy. Three subjects experienced a Grade 3 decrease in blood potassium level and one subject had Grade 2 while being treated with 430-710 mg/kg/day ascorbate. These patients saw their potassium level decrease by a quarter or a third during the study. This incidence of hypokalemia was possibly related to the ascorbate therapy.
This is also supported by our data analysis of the electrolytes after high dose IVC injections for patients who were treated at the Riordan Clinic (not published data). It was shown that frequent high dose IVC injections resulted in the decrease of potassium concentration in blood from 25% to 36%. It is thus recommended to monitor potassium levels during treatment and, if necessary, give oral potassium supplements. Sodium levels were relatively stable during treatment.
Table 3 shows the occurrence of adverse events by IVC dosage. It appears that there are fewer side effects at the lowest dose, although the increases at high doses were not dramatic and did not meet the criterion for stopping the trial.
Table 3. The number of grade 1 – 3 adverse events at each IVC dose. ΣG/ Ns, the sum of grades per subject is given by: ΣG/ Ns = (G1 + 2G2 + 3G3)/Ns where Ns is the number of subjects at that dose.
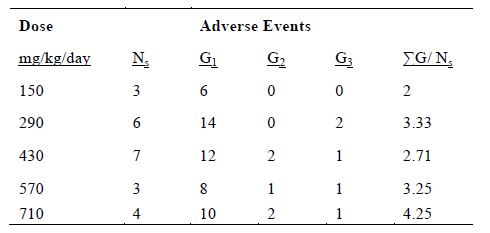
In an attempt to quantify this, the total grades per subject were computed at each dose (Table 3). The results show a trend of more adverse events at higher doses. Grade 2 or 3 hypokalemia was only an issue at the three highest doses tested. Edema was also more frequent in high dosage infusions. There was a single edema event in a total of nine subjects given the two lowest doses of IVC, but there were six cases of edema for patients treated with higher doses. While the highest dose is still safe, the pharmacokinetic data of continuous IVC indicate that the plasma ascorbate concentrations do not increase very much as the dose is increased, so the better strategy may be to use lower doses with longer administration.
DISCUSSION
The purpose of this study was to perform deeper analysis of data from a previously published Phase I clinical trial giving continuous IVC infusions to terminal cancer patients [37]. The primary aim of the Phase I study was to assess risks and determine safety thresholds for IVC doses, particularly in regard to renal function. Efficacy was not expected, and any measures of effects on patient outcome were considered secondary. The eight-week trial involved terminal patients with poor prognosis, all but one of whom showed progressive disease during treatment. However, due to new information concerning potential biological effects of vitamin C potentially relevant to cancer and on the use of white blood cell counts and blood chemistry parameters as prognostic indicators, we decided to examine blood count and chemistry data from this trial to see if any changes in these parameters during IVC therapy could be detected.
The reason for this additional analysis concerns new information concerning IVC therapy in cancer. This includes a more sophisticated understanding of ascorbate’s possible mechanisms of action against tumors. When the clinical trial was conducted, the primary goal was determining whether ascorbate could be administered safely at high doses without compromising renal function, and whether sufficient concentrations could be attained in plasma. The aims of this trial were met.
However, with more recent evidence that IVC could benefit cancer patients through multifunctional mechanisms, the present analysis was conducted to ascertain the effects of continuous IVC on key blood count and chemistry parameters.
In addition, this particular data set allows evaluation of continuous infusion, which would allow the use of lower ascorbate doses while providing longer exposure times at target concentrations. The results of this analysis suggest several potential benefits to continuous IVC infusion.
The most obvious effect of IVC therapy is to increase patient vitamin C levels. Consistent with other reports, the plasma ascorbate measurements conducted in this trial show that vitamin C depletion in cancer patients is common. In fact, ten out of twenty-four subjects entered the trial with plasma ascorbate concentrations undetectable by the colorimetric ascorbate assay used at that time with another four having ascorbate concentrations below the normal range.
IVC infusion increased plasma levels to the order of 1 mM. This likely replenished depleted tissue ascorbate stores as well. Interestingly, there did not seem to be a significant benefit in raising the IVC dosage beyond the first or second dose used, suggesting that for intravenous infusion, there exists a situation analogous to that for oral supplementation in which plasma concentrations reach a saturation point, although the potential saturation point with IV infusion is orders of magnitude higher than what can be attained orally. The leveling off of the ascorbate concentration can be explained by observations that high doses will saturate renal tubular ascorbate reabsorption, leading to increased ascorbate excretion.
Analysis of white blood cell counts for patients in this trial indicate the potential for IVC to increase lymphocyte and neutrophil counts for patients in whom these numbers are below normal
while reducing neutrophil counts in patients for whom ANC counts are elevated.
It was particularly important for lymphocyte counts. Lymphopenia commonly occurs in cancer patients who had chemotherapy and high levels of oxidative stress induced by treatment predicting a poor prognosis [66].
In our study population, about half of the patients who started intervention had ALC lower than normal range. For patients with severe lymphopenia, who completed 6-8 weeks of treatment, the median improvement in the lymphocyte count was 69% and for all patients with ALC lower than normal range the median improvement was 22%. These data proved that continuous IVC can improve immune function of cancer patients by increasing ALC, especially in patients with low lymphocyte count.
The present analysis of neutrophil-to-lymphocyte ratios also demonstrated the regulatory effect of IVC. NLR has been used to assess inflammatory response and has been suggested as a prognostic factor in a variety of cancers [54, 67]. In particular, cut-off values ranging between 2.0 and 4.0 were associated with a significant increase in all-cause mortality [54].
In the present study, most of the patients entered the trial with NLRs well above this cut-off. Continuous IVC therapy tended to decrease the rate of growth of NLR. Moreover, we were able to confirm the predictive potential of NLR: NLR increases correlated with lower survival times. NLR may reflect the balance between the activation of the inflammatory pathway and the anti-tumor immune function. NLR elevated due to neutrophilia is linked to tumor granulocyte colony-stimulating factor (GCSF), accelerated tumor development, and increases in plasma cytokines IL-6 and TNF-α [68]. Since the rate of increase in NLR for patients with initially elevated values decreased during IVC therapy, ascorbate may be decreasing inflammation in these subjects, as suggested in our other work [69]. Also of note is that patients with elevated neutrophil counts tended to have ANC decrease during IVC treatment (while those with lower initial ANC tended to see increases during therapy). Neutrophils may act as tumor-promoting leukocytes [70, 71]. A neutrophilic response is associated with poor prognosis, as it can inhibit the immune system by suppressing the cytotoxic activity of T cells.
In the present study, all but two patients had pre-treatment ANC in normal range. These two patients had above normal ANC initially, but saw their ANC decrease noticeably during IVC therapy. One patient with the initial ANC at the upper end of the normal range had an increase of this count by forty percent during IVC therapy. For the rest of the patients, the tendency was in improving the ANC at the low level of this parameter and decreasing for the higher values. Concentrations of lactate dehydrogenase, an enzyme that catalyzes the conversion of pyruvate to lactate and is thus considered to be a key checkpoint of anaerobic glycolysis, decreased during therapy in 38% of the patients, increased in 28.6% and held constant in 33.4% of patients.
LDH is elevated in many types of cancers; it has been linked to tumor growth, maintenance, and invasion [57]. Among the transcriptional programs turned on by oncogenes, is the stabilization of hypoxia-inducible factor 1 alpha (HIF-1α) [55]. HIF-1α contributes to the upregulation of most of the enzymes and transporters involved in the glycolytic pathway, including lactate dehydrogenase A and glucose transporters, GLUT-1 and GLUT-3 [72]. Since ascorbate is thought to help regulate HIF, it was suspected that IVC might reduce LDH levels, or at least slow down the rate of increase in cancer patients. Data from the present study show an overall decrease in LDH in 40% of patients. In addition, when the subjects are separated into two groups based on initial LDH levels, the group with initial concentrations above the normal range had a significantly lower average survival time than the group with initial LDH levels in the normal range.
Hyperglycemia is another prognostic factor in cancer patients. It is common in cancer patients and represents a challenge during therapy. For example, about 70% of pancreatic cancer patients have impaired glucose tolerance [62]. Moreover, there is a link between the lowering of blood glucose concentration and remission of malignancy. For example, patients under insulin coma therapy for six months (for psychosis) were reported to become free of large tumor burdens considered incurable by their oncologists [62, 73].
Excess glucose may impair cellular dehydroascorbate uptake [74], modify redox balance, activate oxidases, and interfere with the mitochondrial electron transport chain [75]. The elevated glucose levels compete and effectively restrict vitamin C from entering cells. Glucose self-oxidation also leads to free radicals and oxidative stress [74, 75]. In the present study, IVC therapy was associated with decreases in plasma glucose concentrations, consistent with previous reports [76, 77]. This is especially encouraging since two-thirds of the patients were initially hyperglycemic.
Several clinical trials have established that IVC can be administered safely. In the continuous IVC infusion trial from which data for the present analysis are obtained, side effects were mostly minor and the criterion for stopping the clinical trial (two or more Grade 3 or higher adverse events at a given dose at least possibly related to the treatment) was never reached. Adverse events were more frequent at the higher IVC doses used. The adverse event data, along with the pharmacokinetic data, suggest that the use of higher doses in continuous infusion IVC therapy may result in more side effects without increasing plasma ascorbate concentrations significantly beyond those obtained at lower doses.
In summary, despite that the very poor health status of patients and absence of therapeutic efficacy of the treatment showed by radiographic scans, continuous IVC treatment had positive effects on the several important parameters such as NLR, ALC, ANC, LDH and blood glucose concentration. Moreover, these data suggest a therapeutic strategy based on relatively low IVC doses (150 to 290 mg/kg/day, or 10 grams – 20 grams per day for a 70 kg patient) with continuous infusion warrants further consideration.
CONCLUSIONS
Vitamin C is a vital and functional food for presumed health benefits. Humans must obtain vitamin C from the diet, as they do not have gulonolactone oxidase, which is the final enzyme in a sequence of four enzymes in the liver required for vitamin C biosynthesis from glucose.
he use of ascorbate in cancer remains an area of controversy. How intravenous high-dose administration of ascorbate affects tumor growth is unknown and possible mechanisms of antitumor
activity by ascorbate have not been monitored in an in vivo setting. Currently, there are no clinical data that show the importance of several factors in the treatment schedule by high dose
vitamin C, such as dose, frequency and duration of administration on the effectiveness of the cancer patients’ treatment. Most practitioners administer IV ascorbate to cancer patients by bolus
infusions 2-3 times per week. Bolus infusion of high dose ascorbate (1g/kg) can reach very high levels in blood based on the data of pharmacokinetics, but ascorbate is very quickly eliminated
from the body with half life time about one or two hours. Such treatment is generally well tolerated and safe, with few adverse events reported, and there are supporting case reports of an anticancer effect of such regimen.
There are reports regarding anti-tumor effect of relatively lower concentration vitamin C, less than 1.0 mM [78]. In most of the cases, low concentration of vitamin C could not induce extensive apoptosis, but shows the suppression of tumor proliferation and inhibition of the growth factor production [78]. Moreover, there are a number of possible anti-tumor mechanisms that do not require very high concentrations of ascorbate. For example, ascorbate can inhibit hypoxia-inducible factor-1 (HIF-1) activation in vitro at intracellular concentrations between 150 and 300 μM [79]. Pharmacokinetic data on ascorbate in tumor tissues following vitamin C administration determined the optimal dose regimen to achieve cellular levels optimal for HIFhydroxylase activity ~1–3 mM [80]. The study of the optimal concentration of ascorbate as acofactor for hydroxylases that regulate gene transcription and cell signaling pathways shows that ascorbate concentration less than 1,000uM dose-dependently increases 5-hmC signal [81]. Ascorbate availability will influence immune cell function and tumor environments, affecting the resolution of inflammation and potentially tumor survival. The effect of different concentrations of ascorbate on activation of T cells was analyzed in the study [82]. According to this study, at high doses of ascorbate, proliferation was inhibited and there was an increase in apoptosis.
Frequencies of the treatments have effect on mechanisms of tumor suppression. Campbell et al [83] examined the effects of treatment schedule on the ability of intravenous ascorbate to
inhibit HIF-1 expression (and the expression of its target proteins) in tumor bearing mice. As expected, a single bolus injection inhibited expression temporarily (it bounced backed in 20 – 24
hours) while daily injections maintained the inhibition (while also reducing VEGF levels, tumor micro-vessel density, and hypoxia). Increased tumor ascorbate was associated with slowed tumor
growth, but alternate day administration of ascorbate resulted in lower tumor inhibition and did not consistently decrease HIF-1 pathway activity [83].
Clinically, retrospective analysis of prostate cancer patients treated with IVC at the Riordan Clinic (1994-2015) showed that PSA levels increased more slowly in subjects given more frequent IVC treatments [84].
The Riordan Clinic trial patients were treated by continuous infusion, which is usually given over much longer periods of time. The choice of schedule was based on chemotherapeutic regimens, which show that large intermittent doses often being more toxic and less effective than smaller repeated doses.
The study was conducted in 1998 and the first data analysis published in 2005 was focused on the safety of the treatment. We conducted more detailed analysis of the available blood and urine tests and found that continuous IVC infusions improved several parameters associated with poor cancer prognosis.
The present analysis demonstrated the regulatory effect of continuous IVC on neutrophil-tolymphocyte ratios, lymphopenia, neutrophil count and hyperglycemia. The data suggest a strategic benefit of using lower IVC doses in continuous infusions, as raising the dose above 20 grams/70kg body weight increased the frequency of side effects without noticeably increasing plasma ascorbate levels and showed a tendency to decrease improvement in lymphocyte counts. In conclusion, continuous infusions had benefit to cancer patients and further research in this area and clinical studies of the efficacy of continuous intravenous vitamin C are warranted.
Author Contributions: NM and JC analyzed and interpreted data. NM, JC and RH drafted the manuscript. All authors read and approved the final manuscript.
Funding: This research received no external funding.
Acknowledgments: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Levine M, Espey MG, Chen Q. Losing and finding a way at C: new promise for pharmacologic ascorbate in cancer treatment. Free Radic Biol Med 2009, 47, 27–29.
2. Padayatty SJ, Levine M. Reevaluation of ascorbate in cancer treatment: emerging evidence, open minds and serendipity. J Am Coll Nutr 2000, 19, 423–425.
3. Riordan NH, Riordan HD, Casciari JP. Clinical and Experimental Experiences with Intravenous Vitamin C. Journal of Orthomolecular Medicine 2000, 15, 201-213.
4. Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414.
5. Cameron E, Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact 1974, 9, 285–315.
6. Bram S, Froussard P, Guichard M, Jasmin C, Augery Y, Sinoussi-Barre F, Wray W. Vitamin C preferential toxicity for malignant melanoma cells. Nature 1980, 284, 629–631.
7. Casciari JJ, Riordan NH, Schmidt TL, Meng XL, Jackson JA, Riordan HD. Cytotoxicity of ascorbate, lipoic acid, and other antioxidants in hollow fibre in vitro tumours. Br J Cancer 2001, 84, 1544–1550.
8. Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a prodrug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA 2005, 102, 13604–13609.
9. Fujii H, Amano O, Kochi M, Sakagami H. Mitochondrial control of cell death induction by sodium 5,6-benzylidene-L-ascorbate. Anticancer Res 2003, 23, 1353–1356.
10 Jamison JM, Gilloteaux J, Nassiri MR, Venugopal M, Neal DR, Summers JL. Cell cycle arrest and autoschizis in a human bladder carcinoma cell line following Vitamin C and Vitamin K3 treatment. Biochem Pharmacol 2004, 67, 337–351.
11. Leung PY, Miyashita K, Young M, Tsao CS. Cytotoxic effect of ascorbate and its derivatives on cultured malignant and nonmalignant cell lines. Anticancer Res 1993, 13, 475–480.
12. Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA 2008, 105, 11105–11109.
13. Padayatty SJ, Levine M. Vitamin C physiology: the known and the unknown and Goldilocks. Oral Dis 2016, 22, 463–493.
14. Du J, Cullena JJ, Buettner GR. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim Biophys Acta 2012, 1826, 443–457.
15. Fritz H, Flower G, Weeks L, Cooley K, Callachan M, McGowan J, et al. Intravenous vitamin C and cancer: a systematic review. Integr. Cancer Ther 2014, 13, 280–300.
16. Vissers MC, DasFront AB. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Frontiers in physiology 2018, 9: 809.
17. Mandl J, Szarka A, Bánhegyi G. Vitamin C: update on physiology and pharmacology. British Journal of Pharmacology 2009, 157, 1097–1110.
18. Uetaki M, Tabata S, Nakasuka F, Soga T, Tomita M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Scientific Reports 2015, 5: 13896.
19. Kurbacher CM, Wagner U, Kolster B, Andreotti PE, Krebs D, Bruckner HW. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett 1996, 103,183–189.
20. Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, Chen Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med 2011, 50, 1610–1619.
21. Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med 2009, 47, 32–40.
22. Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients 2017, 9: 1211.
23. Mikirova NA, Casciari JJ, Riordan NH. Ascorbate inhibition of angiogenesis in aortic rings ex vivo and subcutaneous Matrigel plugs in vivo. Journal of Angiogenesis Research 2010, 2:2.
24. Mikirova N, Casciari J, Riordan N, Hunninghake R. Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. J Transl Med 2013, 11:191.
25. Duconge J, Miranda-Massari JR, Gonzalez MJ, Jackson JA, Warnock W, Riordan NH. Pharmacokinetics of Vitamin C: insights into the oral and intravenous administration of ascorbate. PRHSJ 2008, 27, 7-19.
26. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr 2011, 2, 78–88.
27. Jackson JA, Riordan HD, Hunninghauke RE, Riordan NH. High dose intravenous vitamin C and longtime survival of a patient with cancer of the head of the pancreas. J Ortho Med 1995, 10, 87-88.
28. Riordan HD, Jackson JA, Riordan NH, Schultz M. High-dose intravenous vitamin C in the treatment of a patient with renal cell carcinoma of the kidney. J Ortho Med 1998, 13, 72-73.
29. Padayatti SJ, Riordan HD, Hewitt SM, Katz A, Hoffer TJ, Levine M. Intravenous vitamin C as a cancer therapy: three cases. CMAJ 2006, 174, 937-942.
30. Drisko JA, Chapman J, Hunter VJ. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. 0J Am Coll Nutr 2003, 22, 118-123.
31. Mikirova N, Hunnunghake R, Scimeca RC, Chinshaw C, Ali F, Brannon C, Riordan N. High-Dose Intravenous Vitamin C Treatment of a Child with Neurofibromatosis Type 1 and Optic Pathway Glioma: A Case Report. American Journal of Case Reports 2016, 10:24.
32. Drisko JA, Serrano OK, Spruce LR, Chen Q, Levine M. Treatment of pancreatic cancer with intravenous vitamin C: a case report. Anti-Cancer Drugs 2018, 29, 373–379.
33. Seo MS, Kim JK, Shim JY. Pulmonary Metastases Originating from Hepatocellular Carcinoma. Yonsei Med J 2015, 56, 1449-1452.
34. Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol 2013, 72, 139-146.
35. Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Annals of Oncology 2008, 19: 2095.
36. Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci 2007, 2, 7-11.
37. Riordan HD, Casciari JJ, Gonzalez MJ, Riordan NH, Miranda-Massari JR, Taylor P, Jackson JA. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J 2005, 24, 269-276.
38. Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo C, Pillai MV, Newberg AB, Deshmukh S, Levine M. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE 2012, 7: e29794.
39. Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med 2014, 6: 222ra18.
40. Polireddy K, Dong R, Reed G, Yu J, Chen P, Williamson S, Violet P-C, Pessetto Z, Godwin AK, Fan F, Levine M, Drisko JA, Chen Q. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Scientific Reports 2017, 7: 17188.
41. Kawada H, Sawanobori M, Tsuma-Kaneko M, Wasada I, Miyamoto M, et al. Phase I Clinical Trial of Intravenous l-ascorbic Acid Following Salvage Chemotherapy for Relapsed B-cell non-Hodgkin’s Lymphoma. Tokai J Exp Clin Med 2014, 39, 111-115.
42. Welsh JL, Wagner BA, van’t Erve T J, Zehra PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts II LJ, Drisko J, Levine M, Buettner GR, Cullen JJ. Pharmacological Ascorbate with Gemcitabine for the Control of Metastatic and Node-Positive Pancreatic Cancer (PACMAN): Results from a Phase I Clinical Trial. Cancer Chemother Pharmacol 2013, 71, 765–775.
43. Hoffman FA. Micronutrient requirements of cancer patients. Cancer 1985, 55(1 Suppl), 295-300.
44. Mayland CR, Bennett MI, Allan K. Vitamin C deficiency in cancer patients. Palliat Med 2005, 19, 17-20.
45. Mahdavi R, Faramarzi E, Seyedrezazadeh E, Mohammad-zadeh M, Pourmoghaddam M. Evaluation of oxidative stress, antioxidant status and serum vitamin C levels in cancer patients. Biol Trace Elem Res 2009, 130, 1-6.
46. Ramaswamy G, Krishnamoorthy L. Serum carotene, vitamin A, and vitamin C levels in breast cancer and cancer of the uterine cervix. Nutr Cancer 1996, 25, 173-177.
47. Fain O, Mathieu E, Thomas M. Scurvy in patients with cancer. BMJ 1998, 316, 1661-1662.
48. Duconge J, Miranda-Massari JR, Gonzalez M, Riordan NR. Schedule-Dependence in Cancer Therapy: What is the True Scenario for Vitamin C? Journal of Orthomolecular Medicine 2007, 22, 1-4.
49. Guichard S, Montazeri A, Chatelut E, Hennebelle I, Bugat R, Canal P. Scheduledependent Activity of Topotecan in OVCAR-3 Ovarian Carcinoma Xenograft: Pharmacokinetics and Pharmacodynamics evaluations. Clin Cancer Res 2001, 7, 3222- 3228.
50. Durand RE, Vanderbyl SL. Schedule Dependence for Cisplatin and Etoposide Multifraction Treatments of Spheroids. Natl Cancer Inst 1990, 82, 1841-1845.
51. Erttmann R, Erb N, Steinhoff A, Landbeck G. Pharmacokinetics of doxorubicin in man:dose and schedule dependence. Cancer Research and clinical Oncology 1988, 114, 509-513.
52. Mallappa S, Sinha A Gupta S, Chadwick SJ. Preoperative neutrophil to lymphocyte ratio > 5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis 2013, 15, 323–328.
53. An X, Ding PR, Wang FH, Jiang WQ, Li YH. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010, 15, 516–522.
54. Socorro Faria S, Fernandes Jr PC, Barbosa Silva MJ, Lima VC, Fontes W, Freitas-Junior R, Eterovic AK, Forget P. The neutrophil-to-lymphocyte ratio: a narrative review. eCancer 2016, 10:702.
55. Miao P, Sheng S, Sun X, Liu J, Huang G. Critical Review. Lactate Dehydrogenase A in Cancer: A Promising Target for Diagnosis and Therapy. 2013 International Union of Biochemistry and Molecular Biology 2013, 65, 904–910.
56. Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin. Cancer Res 2011, 17, 4892–4900.
57. Suh SY, Ahn HY. Lactate dehydrogenase as a prognostic factor for survival time of terminally ill cancer patients: a preliminary study. Eur. J Cancer 2007, 43, 1051–1059.
58. Nelson KA. The cancer anorexia–cachexia syndrome. Semin Oncol 2000, 7, 64–68.
59. Davis MP, Dickerson D. Cachexia and anorexia: cancer’s covert killer. Support Care Cancer 2008, 8,180–187.
60. von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010, 1, 1–5.
61. Sargeant LA, Wareham NJ, Bingham S, Day NE, Luben RN, Oakes S, Welch A, Khaw KT. Vitamin C and hyperglycemia in the European prospective investigation into cancer—Norfolk (EPIC-Norfolk) study: a population-based study. Diabetes Care 2000, 23, 726–732.
62. Krone CA, Ely JTA. Controlling hyperglycemia as an adjunct to cancer therapy. Integer. Cancer Ther 2005, 4, 25-31.
63. Blanchard J, Tozer TN, Rowland M. Pharmacokinetic perspectives on megadosis of ascorbic acid. Am J Clin Nutr 1997, 66, 1165-71.
64. Mackall, CL, Fleisher, TA, Brown, MR, Magrath, IT, Shad, AT, Horowitz, ME, Wexler, LH, Adde, MA, McClure, LL, Gress, RE. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994, 84, 2221-2228.
65. Verma R, Foster RE, Horgan K, Mounsey K, Nixon H, Smalle N, Hughes TA, Clive RD. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Research 2016, 18: 10.
66. Lee CK, Gurney H, Brown C, Sorio R, Donatello N, Tulunay G, Meier W, Bacon M, Maenpaa J, Petru E, Reed N, Gebski V, Pujade-Lauraine E, Lord S, Simes RJ, Friedlander M. Carboplatin–paclitaxel-induced leukopenia and neuropathy predict progression-free survival in recurrent ovarian cancer. British Journal of Cancer 2011, 105, 360 – 365.
67. Liu D, Huang Y, Li L, Song J, Zhang L, Li W. High neutrophil-to-lymphocyte ratios confer poor prognoses in patients with small cell lung cancer. BMC Cancer 2017, 17:882.
68. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013, 19, 1423–37.
69. Mikirova N, Casciari J, Taylor P, Rogers A. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. Journal of Translational Medicine, 2012, 10:189.
70. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013, 218, 1402–10.
71. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 2012, 22, 33–40.
72. Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434.
73. Koroljow S. Insulin coma therapy. PsychiatrQ 1962, 36, 261-270.
74. Chen L, Jia RH, Qiu CJ, G. Ding G. Hyperglycemia inhibits the uptake of dehydroascorbate in tubular epithelial cell. Am J Nephrol 2005, 25, 459-465.
75. Ruhe RC, McDonald RB. Use of antioxidant nutrients in the prevention and treatment of Type 2 diabetes. Journal of the American College of Nutrition 2001, 20, (supplement 5), S363– S383.
76. Dakhale GN, Chaudhari HV, Shrivastava H. Supplementation of Vitamin C reduces blood glucose and improves glycosylated hemoglobin in Type 2 Diabetes Mellitus: A Randomized, Double-Blind Study. Advances in Pharmacological Sciences 2011, 2011:195271.
77. Ardekani MA, Mohiti J, Amirchaghmaghi E, Modarresi M. The effect of vitamin C supplementation on insulin level, HbA1c and blood glucose in type 2 diabetic patients. Journal of Kerman University of Medical Sciences 2006, 11, 12–18.
78. Yu Y, Bae S, Kim H, Kim Y, Chu NB, Nag Kyun Chu NK, Kang JS, Lee WJ. The Antitumor Activity of Vitamin C via the Increase of Fas (CD95) and MHC I Expression on Human Stomach Cancer Cell Line, Immune Network, 2011, 11: 210
79. Kuiper C, Dachs GU, Munn D, Currie MJ2, Robinson BA, Pearson JF, Vissers MCM. Increased tumor ascorbate is associated with extended disease-free survival and decreased hypoxia-inducible factor-1 activation in human colorectal cancer. Front. Oncol 2014, 10, 4:359|
80. Myllyla R, Kuutti-Savolainen ER, Kivirikko KI. The role of a ascorbate in the prolylhydroxylase reaction. Biochem Biophys Res Commun 1978, 83, 441–8.
81. Minor EA, Court BL, Juan I. Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Bio Chem 2013, 288, 13669-74
82. Hong JM, Kim JH, Kang JS, Lee WJ, Hwang Y. Vitamin C is taken up by human T cells via sodium-dependent vitamin C transporter 2 (SVCT2) and exerts inhibitory effects on the activation of these cells in vitro. Anatomy and cell Biology, 2016, 49, 88-98
83. Campbell EJ. Vissers MCM, Wohlrab C. Kevin 0. Hicks KO, Strother RM, Stephanie M. Bozonet SM, Robinson BA, Dachs GU. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radical Biology and Medicine 2016, 99,151-462
84. Mikirova N, Hunninghake R. Changes in the rate of PSA progression and the level of alkaline phosphatase during high dose vitamin C treatment of patients with prostate cancer. Functional Foods in Health and Disease 2017, 7, 511-528.




