Changes in the rate of PSA progression and the level of alkaline phosphatase during high dose vitamin C treatment of patients with prostate cancer
Riordan Clinic, Bio-communication research institute, Wichita, KS, USA
Corresponding Author’s information: Nina Mikirova, Address: 3100 North Hillside, Wichita,
KS, USA
Submission date: May 3rd, 2017, Acceptance Date: July 24th, 2017, Publication Date: July 31st, 2017
Citation: Mikirova N., Hunninghake R., Changes in the rate of PSA progression and the level of
alkaline phosphatase during high dose vitamin C treatment of patients with prostate cancer.
Functional Foods in Health and Disease 2017; 7(7): 511-528
ABSTRACT
Introduction: Intravenously administered vitamin C (IVC) may have anti-cancer and antiinflammatory
properties. Many studies demonstrated evidence of a good safety profile of IVC
treatments and improvement of the quality of life in cancer patients. IVC has been proposed as a
treatment for cancer as an adjuvant in conjunction with other therapies. To investigate high dose
ascorbic acid potential in treating prostate cancer, a retrospective study was conducted using
clinical data from the Riordan Clinic database (1994-2015).
Methods: We collected data, when available, on the following patient characteristics at diagnosis
and during the courses of IVC therapy: age, tumor stage, Gleason score, serum prostate specific
antigen (PSA) and alkaline phosphatase (ALP) levels, and location of metastases. In particular,
PSA, ALP, and C-reactive protein (CRP) levels are analyzed in prostate cancer patients given IVC
therapy during several years.
Results: We found that PSA, CRP, and ALP correlate with tumor staging as measured by Gleason
scores. Moreover, peak plasma ascorbate levels attained during the patients first IVC infusions are
reduced in patients with elevated PSA and CRP levels. Tracking the changes in PSA and ALP with
time in patients for whom data are available indicates that the rate of increase in these variables
over time can be reduced by incorporating IVC therapy and by increasing the frequency of IVC
treatments.
Functional Foods in Health and Disease 2017; 7(7): 511-528 Page 512 of 528
Conclusion: There appeared to be a relation between the frequency of IVC treatments and the
rate of PSA change, with PSA rate of growth decreasing as the frequency of IVC increases. Further
research into the use of IVC in prostate cancer patients is warranted.
Key words: High dose vitamin C, prostate cancer, prostate specific antigen, alkaline phosphatase,
C-reactive protein.
INTRODUCTION
Vitamin C (ascorbic acid, AA) is an essential water-soluble antioxidant that plays significant roles
in immune function, mediation of inflammation, and tissue repair via collagen production [1]. It
has been studied extensively for its potential in preventing chronic diseases [2].
Intravenous high dose vitamin C (IVC) is a commonly used therapy among naturopathic
doctors and other integrative oncology healthcare practitioners. Many studies demonstrated
evidence of a good safety profile and potentially important anti-tumor activity of high-dose vitamin
C [3-6]. IVC treatments have been reported to improve the quality of life in cancer patients [7, 8]
and have been proposed as a treatment for cancer as an adjuvant in conjunction with other therapies
[9-11].
There are several potential motivations for using IVC in cancer therapy. For instance, cancer
patients are often deficient in vitamin C, and this deficiency is correlated with decreased dietary
intake, increased inflammation, and reduced survival time [12].
The anticancer mechanisms of vitamin C are not fully understood. Several mechanisms were
proposed such as the effects of high doses of vitamin C on angiogenesis, suppression of
inflammation, modulation of gene expression, and improvement of immune cells’ functioning [13-
16]. There are in vitro and animal studies that showed that high-dose vitamin C induces pro-oxidant
effects and selectively kills cancer cells [17, 18]. Increased reliance of cancer cells on glycolysis
suggested that following IVC treatment elevated uptake of oxidized ascorbic acid may cause
oxidative stress by depleting glutathione [19], as well as the mechanism of extracellular delivering
of hydrogen peroxide to tumor tissue.
As ascorbate has been shown to stimulate the production of type I and III collagen [20], the
stimulation of collagens’ production in the extracellular matrix can be important components of
the physical barrier against invasion and metastasis of cancer cells.
Recent studies indicate that vitamin C may have important effects on gene expression. Since
the induction of HIFs may be mediated by ROS, antioxidants such as vitamin C may inhibit HIF-
1 and HIF-1a expression; this is supported by experimental studies [21]. Vitamin C may also act
on HIF-1 expression by inhibiting the expression of Nuclear Factor kB (NF-kB) genes. NF-kB is
involved in inflammation and tumor development [22]. Ascorbate has been shown to inhibit the
expression of NF-kB [23, 24]. The anticancer effect of ascorbate may be in part due to its ability
to suppress specificity protein (SP) transcription factors [25]. Sp-regulated genes are involved in
cancer proliferation, survival, and angiogenesis. Ascorbate was also shown to suppress tumor
growth through the modulation of p53 [26]. This creates a rationale for using high dose ascorbate
as an anti-tumor agent.
Oxidative stress and inflammation are key developments in prostate cancer progression, and
the strong correlation between C-reactive protein, an inflammation marker, and prostate specific
Functional Foods in Health and Disease 2017; 7(7): 511-528 Page 513 of 528
antigen, a cancer marker, suggests that chronic inflammation may be a legitimate target in prostate
cancer treatment [27]. Inflammation is fundamental in prostate cancer: evidence implicates it in
prostate cancer development and progression to advanced metastatic disease [28]. In many
cancers, inflammatory gene markers are correlated with poor prognosis [29].
IVC therapy reduced CRP levels and decreased plasma concentrations of several
inflammatory and angiogenic cytokines; moreover, these decreases correlated with reductions in
the levels of tumor markers [15, 30]. Prostate cancer may be a particularly promising candidate
for IVC therapy. Several studies show prostate tumor cells in vitro, in addition to implanted
prostate tumors in animals, are sensitive to ascorbate [31-34]. Moreover, a clinical study
combining vitamin C and vitamin K3 in prostate cancer patients who failed standard therapy
showed promise in delaying biochemical progression, as measured by decreases in PSA velocity
and doubling time [35].
Since the Riordan Clinic has been treating cancer patients with IVC for decades, we have a
potentially useful database of patients with prostate cancer and IVC. In the present manuscript, we
examined our historic data for prostate cancer patients given IVC, with a particular focus on
tracking changes in PSA levels and—where data are available—alkaline phosphatase levels. PSA
is a reliable, sensitive, easy to measure, and widely used biomarker for prostate cancer. There is
some indication that declines in PSA levels are predictive of longer patient survival [36-38]. ALP
is particularly useful as an indicator of osteoblastic activity [39], and may in fact be a better
predictor of survival than PSA [40].
METHODS
We analyzed the data of prostate cancer patients from the Riordan Clinic (Wichita, KS) who were
treated with IVC and who had PSA and ALP levels measured at some point before and after
treatment.
The study was conducted under Institutional Review Board Approval of Riordan Clinic.
Demographics were limited to ensure confidentiality.
Using the LabNet laboratory management program (Henry Schein, Melville, NJ), we collected
data from 180 prostate cancer patients when available, on the following patient characteristics at
diagnosis and during the courses of IVC therapy: age, tumor stage and grade at diagnosis, Gleason
score, serum PSA and ALP levels, location of metastases (if present), and survival time (time from
diagnosis to death). Note that not all of these parameters were available in the database for all
patients: for example, Gleason scores were only available for roughly half of the subjects, and
many of the patients were lost to follow up. Averages for available values are given in Table 1,
along with the percentages of patients who had conventional treatments and the survival times.
Most of the patients were treated by IVC after conventional treatment: surgery, chemotherapy,
or radiation. 16 patients refused conventional treatment and choose alternative treatment. For all
patients treated by IVC the periods of analysis were chosen when patients did not have
conventional treatment.
Obviously, in a retrospective study, such as one covering 20 years of research, protocols and
doses varied from subject to subject. However, in general IVC was administered in accordance
with the Riordan IVC protocol [5]. Briefly, new cancer patients were given a 15-gram ascorbate
infusion for their first dose, followed by a 25 gram infusion on day two. Dosage was then gradually
Functional Foods in Health and Disease 2017; 7(7): 511-528 Page 514 of 528
adjusted upward by the physician, based on patient tolerance for the treatment and the plasma
ascorbate levels attained, to maximum doses between 50 grams and 100 grams. Frequencies of
treatments ranged from several times per week to once-per-month.
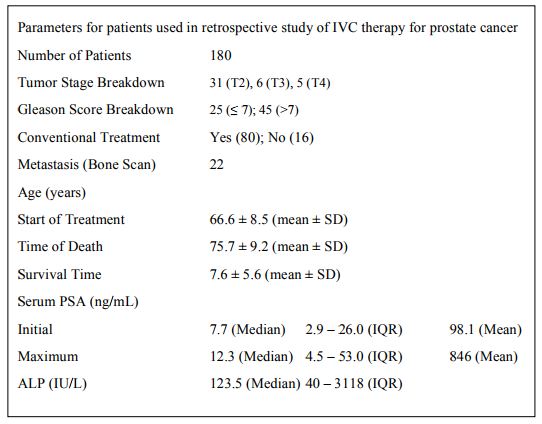
Table 1. Parameters for patients used in retrospective study of IVC therapy for prostate cancer
All laboratory tests were conducted by the Riordan Clinic Bio-Center Laboratory, a licensed
and certified medical laboratory working in full compliance with HIPPA regulations and using
standard methodologies. Plasma ascorbate concentrations were measured by HPLC with
electrochemical detection. Serum PSA concentrations were determined using an equimolar
immunoassay (PSA kit, Abbott Laboratories, UK). According to this protocol, the upper normal
range for PSA is 4.0 ng/mL. Serum ALP was measured using a standard commercially available
assay, with the normal range in our laboratory being 30U/L-110 U/L.
Statistical analyses were carried out using the Excel spreadsheet program, while graphs with
the least squares regression data fits where appropriate were constructed using the Kalaidagraph
program (Synergy Software, Reading PA).
RESULTS
Our database contained a total of 180 prostate cancer patients. However, complete information on
the tumor stage, presence or absence of metastases, and history concerning the use of conventional
therapies were not available for many of these subjects. The effect of the tumor stage on the values
of two key cancer markers PSA and ALP is shown in Figure 1. Increases in these markers with
clinical stage is shown here, in addition to that of metastases, and are as expected based on prior
reports.
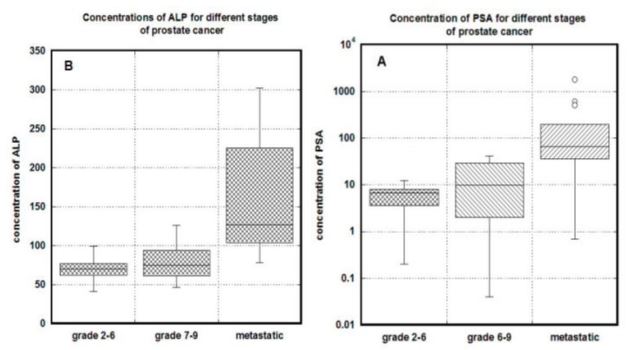
Figures 1 (A, B). Relationships between tumor stage and serum concentrations of PSA (A) and
ALP (B). The stages of prostate cancer were divided by Gleason scores of 6 or less, without
metastases; Gleason scores of 7 or more, without metastases; Gleason Scores of 7 or more, with
metastases. Box plots indicate quartile ranges (1st quartile to 3rd quartile in box, with line inside
box representing a median value).
The distributions of PSA for different states of diseases are shown in Figure 1A. PSA
increased with an increasing clinical stage, and patients with metastasis had a statistically higher
level of PSA than patients with localized tumor (P<0.001). Distributions of ALP for different
Gleason scores and localized or metastatic diseases are shown in Figure 1B. According to these
data the significant increase in ALP was measured for metastatic state of the cancer (p<0.03). ALP
averages were 70±14 U/L for grades 2-7, 79±24 U/L for grades 7-9 and 196±127 U/L for
metastatic stage of disease.
For many subjects, ascorbate pharmacokinetic data were available. Concentration of ascorbic
acid was measured in plasma before and after IVC. High doses of injected ascorbic acid
intravenously produced the average peak plasma AA concentrations ranged from 5 mM (88
mg/dL) at dosage 15 grams to the levels 10 mM (176 mg/dL) and 14 mM (246 mg/dL) at dosages
25 grams and 50 grams [5]. Typically with IVC, the elimination half-life at such dosages is
roughly two hours [41].
Initial IVC treatments (15 gram dose, infused over a one-hour period) led to peak plasma
ascorbate concentrations in cancer patients that varied considerably from patient to patient. As
shown in Figure 2, patients who had lower PSA levels, or lower CRP levels, attained higher peak
plasma ascorbate concentrations
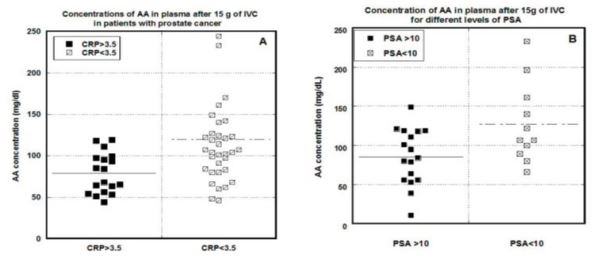
Figures 2 (A, B). Relationships between CRP (A) or PSA (B) concentrations and the peak plasma
ascorbate concentration attained in the patient’s first IVC infusion. PSA and CRP concentrations
separated to less than 10 ng/mL for PSA or less than 3.5 mg/L for CRP or above those thresholds (p<0.002
for CRP and p<0.03 for PSA). Lines represent median values.
These differences were statistically significant (p < 0.002 for CRP and p < 0.030 for PSA),
and are consistent with earlier studies indicating that cancer patients have lower peak ascorbate
levels compared to healthy adults, and the achievable levels of AA in plasma decreases with
increased levels of inflammation and the progression of disease [42].
Based on the idea that conditions like cancer and chronic inflammation lead to lower ascorbate
levels and higher levels of oxidative stress, it makes sense that subjects with a higher tumor burden
(as indicated by PSA) or a greater degree of chronic inflammation (as indicated by CRP) would
have lower ascorbate levels. Subsequent IVC treatments at the same dose typically yield lower
plasma ascorbate concentrations as depleted tissue stores are replenished.
The overall efficacy of IVC against cancer for all prostate cancer patients, as measured by
PSA or CRP levels, was difficult to evaluate for a variety of reasons. In some cases, patients
discontinued treatments after a relatively short time from the beginning. In others, key lab
parameters (such as PSA) were not adequately tracked. Moreover, patients varied considerably in
terms of what other treatments (conventional or alternative) they received in addition to IVC.
There were some subjects who were treated with IVC for a regular and prolonged period and
for whom PSA levels were monitored at regular intervals. In these subjects at the periods when
they did not have conventional treatment, we were able to analyze how IVC treatments affected
PSA levels and PSA velocity. In doing so, we define the PSA velocity (PSAV), the change in PSA
concentration per day between measurements, and the IVC treatment frequency (IVCf), the
number of IVC treatments per day between PSA measurements:
• PSAV = (PSA2 – PSA1) ÷ (t2 – t1) units of ng/mL/day
• IVCf = {Number of IVC treatments over interval t1 – t2}÷(t2 – t1) units of day-1
An example of how these variables tracked each other in one patient (denoted “Patient 1”) is shown
in Figures 3A – 3C. Data in Figure 3 show PSA concentration (ng/mL) versus time along with
indications of IVC treatments in grams, the change in PSA concentration, and IVC treatments per
day, in addition to correlate]on between PSAV and IVCf. The data points were approximated by
regression line: y = 0.49 – 6.9x+23.1×2 (r = 0.66).
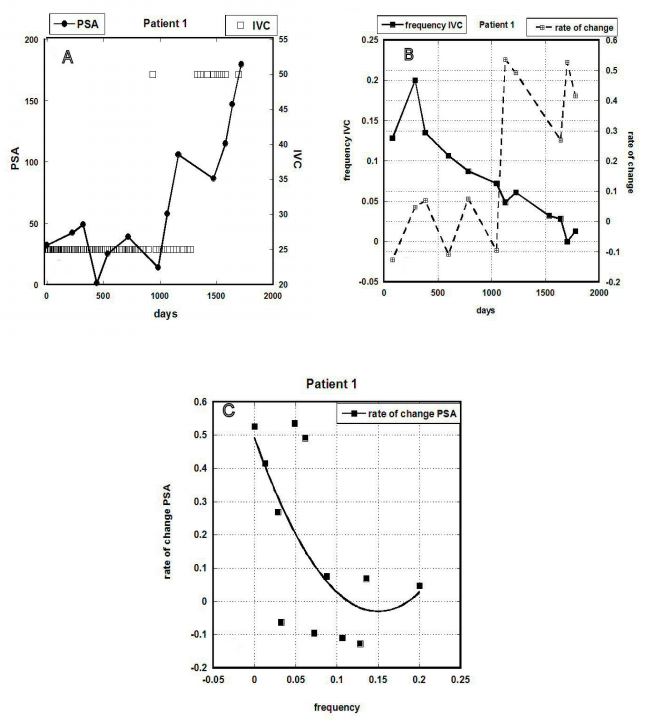
Figure 3 (A-C). Data for Patient 1: (A) PSA concentration (ng/mL) versus time, along with
indications of IVC treatments in grams given. (B) The rate of PSA concentration change and
frequency of IVC (treatments per day) versus time. (C) Correlation between PSAV and IVC
frequency (r=0.66).

Figure 4 (A-F). PSA concentration, the rate of PSA change, IVC treatments and the frequency of
IVC treatments versus time (days) for Patients 2 through 7. Squares on the right Y-axis indicate
dosage of IVC treatments and dark circles on left Y-axis—the measured levels of PSA. Values in
inserts show the rate of PSA change (right Y-axis) and the frequency of IVC treatment (left Yaxis).
This patient (Patient 1, Figure 3) came to the clinic in 2006. A biopsy performed six months
before his visit showed a stage D2 castration resistant tumor with a Gleason score of 8. The patient
did not have surgery or hormonal treatments (choosing exclusively homeopathic treatments
instead). IVC therapy was first carried out in 2006 and stopped in 2011. In 2012, when the tumor
was discovered to be metastatic, the patient began Casodex and Lupron treatments and had surgery
(TURP). In 2014, he was placed in clinical trials for Cabozantinib and Taxotere.
Data presented in Figure 3 are related to the period of IVC treatment before chemotherapy
and surgery. According to these data for the period about five years, the patient was treated by 150
IVCs with dosages 25 grams and 50 grams with frequencies one or 1.5 times per week, one time
per two weeks or at the end of the treatment, one time per month.
It appears from Figure 3A that PSA levels fluctuated without significant increase at first
(during the first thousand days) and then began to increase more rapidly. The number of treatments
was reduced at this point, as shown in Figure 3B. It can be seen here that as the number of
treatments was reduced (and even after the dose per treatment was increased), the PSA
concentration rose. The correlation between these two variables is shown in Figure 3C, suggesting
that for this patient a reduction in the number of treatments corresponded to higher PSAV values.
As a result, the frequency of the treatment the rate of growth of PSA was also changed and
increased for less frequent treatments.
Figure 4 shows the tracking of PSA and rate of PSA growth with the frequency of IVC
treatments (IVCf) for six other patients (Patients 2 through 7). Inserts in each graph present the
same information as in Figure 3B (the dependence with time frequency of IVC on the first Y-axis
and rate of PSA change on the second Y-axis). In most of the cases, the decrease in the frequency
of the IVC resulted in the increase of the rate of growth of PSA.
A description of each patient is also given:
• Patient 2 (Figure 4A) came to the clinic at age 61, his tumor having been evaluated four
years earlier with a Gleason score ranging from 6 to 9 (depending on area). The records in
the database showed that the patient did not have metastases and did not use conventional
therapy. This patient was followed for over seven years, and had more than 230 IVC
treatments during this time. For the first three years, he received doses of 75 grams to 100
grams IVC once per week. In the last four years, he was given 25 grams IVC one or two
times a week. There were several intervals of roughly a month in duration each during
which he received no treatments. The average rate of PSAV changed from 0.19±1.27
ng/mL/day for periods with frequency of injection one per week or higher to 0.52±2.15
ng/mL/day for frequencies of IVC less than one per week. Additionally, for the periods of
treatment when the treatment by IVC was even less frequent, the rate of PSAV was
increased to 1.26±2.61 ng/mL/day. There were no records of the conventional treatments
at the end of the analyzed period, when the PSA level declined.
• Patient 3 (Figure 4B) had transurethral resection of prostate (TURP) five years before he
came to the clinic for IVC treatment. He had a Gleason score at diagnosis of 4 and elected
nutritional therapies, refusing surgery and radiation. During the next half year he had 42
IVC treatments (25 grams IVC each), with one treatment per 5 days on average. PSAV
decreased from 0.38 ng/ml/day to 0.03 ng/ml/day, and PSA concentration was reduced
from 41 ng/mL to 5 ng/mL. During treatment the patient said that he had the best sleep he
had in many years. Patient continued treatment for an additional three months and then
stopped treatment. One month after the discontinuation of the treatment, he complained
about increased pain and his serum PSA increased to 71ng/mL.
• Patient 4 (Figure 4C) was followed for roughly two years. There are no data for this patient
concerning tumor stage or the use (or not) of conventional therapy before IVC treatment,
probably because of the tumor was early stage. During the first year we followed, he
received 29 IVC treatments (with dosages of 15, 25, or 50 grams each). PSA levels were
stable during this time between 6 ng/mL to 7 ng/mL. The patient then discontinued
treatments. From this point next measurement of PSA after 10 months showed that PSA
levels doubled.
• Patient 5 (Figure 4D) came to the clinic in 2007 with a Stage 2 T1cN0M0 tumor that had
a Gleason score of 5. He did not have conventional therapy, but instead received IVC
treatments. After initial 15 g and 25 g infusions, he received ten treatments of IVC 50 grams
each, at a frequency of one or two times per week. During this time period, PSA
concentrations remained stable. The patient then discontinued treatment for roughly 1.5
years, experiencing a doubling of PSA concentrations during this time. IVC treatments
were then resumed at 50 grams IVC each. This treatment resumption corresponded to a
stabilization of the PSA level (a drop in PSA levels from 11 ng/mL to 8 ng/mL over a fourmonth
period).
• Patient 6 (Figure 4E) was diagnosed with prostate cancer at age 68. The tumor stage at the
first visit was: Gleason Score 6, T1cN0M0. There is no record of this patient receiving
conventional therapy during the five year period during which he had a total of 57 IVC
treatments. The analysis of the rate of PSA growth with the frequency of the treatment
showed that at the highest frequency of the treatment (once per week) the rate of PSA
growth was at level zero and increased at less frequent treatments. This patient’s PSA levels
generally increased during the five year period over which we have data, but periods of
low PSA increase corresponded to higher IVC treatment frequencies.
• Patient 7 (Figure 4F) came to the Riordan Clinic after previously receiving conventional
treatment: radiation and two times chemotherapy (Lupron) during four months and one
month with three-year interval between treatments. Records indicate that the tumor was
not considered malignant at that time, but PSA levels were rising dramatically prior to
beginning IVC therapy. The PSA after the last treatment by Lupron decreased to the level
14 ng/ml but then started to increase. The patient came to the clinic and started IVC
treatment one and half year after the last conventional treatment when the PSA increased
to the level 360 ng/mL. During the analyzed period, patient had 24 IVC with high dosages
of 50-75 grams. The rate of PSA growth was suppressed when patient had one IVC per day
and increased about three times at the frequency one IVC per month.
The later data showed that after IVC treatment patient was treated by Casodex during one
month, the level of PSA stabilized but increased more than 15 times two months after the end of
the treatment.
The correlations between the rate growth of PSA and frequency of treatments is shown in Figure
5, with data from a total of fifteen patients for whom detailed PSA concentration vs. time data
were available.
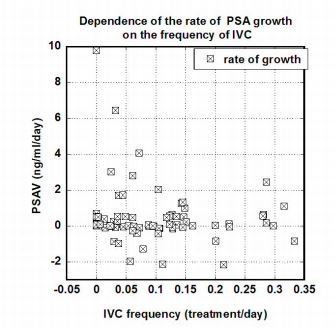
Figure 5. Correlation between PSAV (ng/mL/day) and the frequency of IVC treatments
(treatments/day) for fifteen prostate cancer patients.
These data show decreases in the rate of PSA concentration growth with increased IVC
treatment frequencies. The correlation was not significant at the 0.05 level. For instance, in
comparing IVC frequencies of once per week with lower frequencies, the p value was roughly 0.16
and was reduced to 0.06 when values for PSA concentrations above 20 ng/mL were considered.
The high variability of the data can be explained by the different stage of the disease of different
patients, as the effectiveness of treatment may depend on many factors including the
aggressiveness of tumor, initial levels of PSA and inflammation, and the state of immune system.
Ultimately, a systematic study is needed, but the data in Figures 3 through 5 suggest that PSA
levels are generally less likely to increase when IVC is administered often and sufficiently.
The other parameter we investigated in detail was ALP, a marker of bone formation that can
be used to some degree to track bone metastases in prostate cancer patients. Alkaline phosphatase
is one of the older biochemical toolsfor investigating and monitoring prostate cancer, and a reliable
indicator of osteoblastic activity and bone metastases.
Since this was not measured nearly as often as PSA, we were confined to examining three
subjects who had described ALP vs. time data. The data for these subjects are shown in Figure 6,
with descriptions of each patient (Patient 3 described above, in addition to Patients 8 and 9).
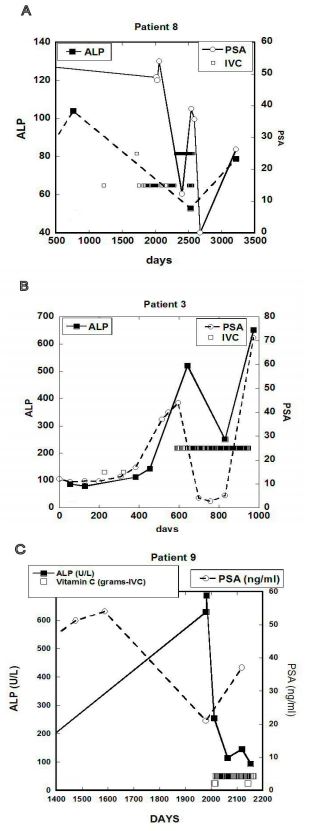
Figure 6 (A-C). ALP concentration (IU/L) and the PSA concentration (ng/mL) for three cancer
patients. Concentrations of PSA are shown by open circles on the right Y-axis and concentrations
of ALP are shown by dark squares on the left Y-axis. Open squares indicate times when IVC
treatments were given and dosages in grams on Y-axis according to labels.
In these patients, the onset of IVC therapy, and the increasing of its frequency, led to
reductions in ALP values. PSA levels also tended to track APL levels for these subjects.
• Patient 3 (Figure 6A) showed ALP increases from 149 U/L to 512 U/L in the six months
prior to treatment. After seventy-six IVC treatments at 15 g each, the ALP concentration
decreased to 251 U/L. This also correlated with a decrease in PSA. One month after the
discontinuation of IVC therapy, concentrations of PSA and APL showed sharp increases.
• Patient 8 (Figure 6B) came to the clinic in 1994 with diagnosis of prostate cancer. He
refused biopsy, bone scan, and conventional therapy. The patient was treated with 15 grams
IVC at a frequency of roughly once per week. During the first year of treatment, his PSA
concentration decreased from 54 ng/mL to 12 ng/mL, and eventually was reduced to
normal levels after another year of treatment. One year without treatment saw his PSA
levels increased to 26 ng/mL. ALP levels over time decreased from an initial level of 105
U/L to 53 U/L after 80 IVC treatments and increased to 73 U/L one year after
discontinuation of treatment.
Patient 9 (Figure 6C) first came to the Riordan Clinic having a “hard and nodular” prostate.
He did not have a biopsy and refused chemotherapy. Seven years later, metastasis was
diagnosed in the liver and bone. At this time, the patient’s ALP concentration was 687 U/L.
The patient had surgery (cholecystojejunostomy & gastrojejunostomy) prior to starting
IVC therapy. Two weeks post-surgery ALP concentration was reduced to 255 U/L. At this
point, the patient started 50 gram IVC treatments two times per week. Two months later,
the level of ALP decreased to 155 U/L. After 3 months and 24 IVCs ALP decreased further
to 94 U/L. During treatment with IVC the patient recorded that he was feeling a lot better.
PSA concentrations ranged from 21 ng/mL to 37 ng/mL.
Altogether, these three cases show dynamic changes in alkaline phosphatase during IVC
therapy. As ALP suppression is thought to indicate suppression of osteoblast bone formation in
prostate cancer patients, the ALP decreases with IVC treatment are encouraged if preliminary.
DISCUSSION
In an effort to develop effective alternative strategies that increase the therapeutic efficacy and
minimize the systemic toxicity of chemotherapeutic agents, many efforts are being directed
towards the investigation of nutraceutical agents and in particular high dose vitamin C as a
therapeutic agent.
The purpose of this study was to determine if IVC therapy could suppress PSA and ALP levels
in prostate cancer patients. Our analysis of the Riordan Clinic cancer database indicates that this
may well be the case, and suggests that further systematic clinical studies into this phenomenon
are warranted. Our conclusions can be summarized as the following:
1. Consistent with earlier studies, we find that PSA, ALP, and CRP concentrations correlated
with Gleason scores and the presence of metastasis in our patient group.
2. In cases where pharmacokinetic data are available, we found that patients with higher
Gleason scores and/or with metastases attain lower plasma ascorbate levels with a given
dose infusion. This is consistent with earlier reports that cancer patients are deficient in
vitamin C and that this deficiency is more pronounced as their disease progresses.
3. There appears to be a correlation between the frequency of IVC treatments and the PSA
“velocity,” with PSAV decreasing as IVC frequency increases, although we were not able
to demonstrate statistical significance at the 0.05 level.
4. In the few cases where we found both PSA and ALP measurements recorded, these
variables tended to track each other, and they tended to both decrease during IVC therapy.
5. In several cases, the reductions in PSA and/or ALP concentrations (or their stabilization)
were reversed once the treatment stopped.
As the PSA concentration varies depending upon tumor differentiation, tumor volume, and
the extent of disease [37, 38], the relationship between the PSA rate of change and frequencies of
IVC treatment may indicate some inhibitory effect of the treatment on the cancer. PSAV, the rate
of change in concentration of the PSA marker, is thought to be an important prognostic parameter.
Several reports suggest that PSA doubling time can be used to predict the aggressiveness of the
disease [43], and may be useful in identifying patients at risk for progression after prostatectomy
or radiation therapy.
Our preliminary observations concerning IVC therapy and ALP levels were encouraging.
According to a large multivariate analysis of patients with metastatic androgen-independent
prostate cancer, elevated serum ALP levels were independently associated with shorter survival
[44]. Moreover, ALP levels have been associated with the progression of skeletal metastases in
patients with prostate cancer [45] and have also been shown to be significant predictors of early
death [46, 47]. While osteoclastic processes are seen as a potential target for prostate cancer
therapy, chemotherapeutic drugs aimed at inhibition these processes offer only a few months
advantage over placebo in prolonging survival time [48], and often carry side effects such as
osteonecrosis of the jaw, hypocalcemia, and deterioration of renal function [49]. IVC therapy is
unlikely to have such severe side effects, and may act as an adjuvant to reduce side effects of other
agents. Furthermore, ascorbic acid induces formation of collagen matrix and enhances osteoblast
differentiation [50]. Since cancer patients are ascorbate deficient and have an abnormal demand
for ascorbate due to oxidative stress, using AA as an adjuvant to support these processes may help
prevent metastasis in prostate cancer patients.
CONCLUSION
Our study is the first to address dynamic changes of the easily available biomarkers ALP and PSA
during alternative therapy by high dose IVC. We underlined the possibility of ALP decline, which
is a marker of suppression of osteoblast bone formation marker, suppression of the rate of PSA
growth, and the dependence of the suppression of the rate of PSA growth on the frequency of the
high dose vitamin C treatment. Both these dependencies resulted from the analysis of the data,
demonstrating the clinical benefit of IVC for prostate cancer patients.
This study was ultimately limited by the small number of patients in our database who had
sufficiently detailed information about analyzed parameters, frequent PSA, and ALP
measurements, in addition to consistent participation in the IVC protocol. A controlled study using
a larger population of prostate cancer patients would allow for a more rigorous assessment of the
promising trends reported in this study.
List of abbreviations: prostate specific antigen (PSA), intravenous vitamin C (IVC), alkaline
phosphatase (ALP), C-reactive protein (CRP).
Competing interests. The authors declare that they have no competing interests associated with
this manuscript.
Authors’ contributions: Nina Mikirova, PHD, is the principle investigator of this study and
contributed to design, data analysis, statistical analysis and writing of the manuscript. Ronald
Hunninghake, MD, assisted in interpretation of the results.
Acknowledgement and funding: The authors thank the Riordan clinic laboratory for performing
clinical tests. The funding of the study was provided by the Riordan Clinic.
REFERENCES
1. Jariwalla RJ, Harakech S. “Ascorbic acid,” in Biochemistry and Biomedical Cell Biology,
J. R. Harris, Ed., , New York, NY, USA, Plenum Press, 1996. pp. 215–231.
2. Levy TE. Primal panacea. MedFox Publishing; 2012.
3. Jacobs C, Hutton B, Ng T, Shorr R and Clemons M. Is There a Role for Oral or
Intravenous Ascorbate (Vitamin C) in Treating Patients With Cancer? A Systematic
Review. The Oncologist. 2015; 20: 210-23.
4. Ohno S, Ohno Y, Suzuki N, Soma GI and Inoue M. High-dose Vitamin C (Ascorbic
Acid) Therapy in the Treatment of Patients with Advanced Cancer. Anticancer
Research. 2009; 29:809-816.
5. Riordan H, Riordan N, Casciari J, Jackson J, Hunninghake R, Mikirova N, Taylor P. The
Riordan intravenous vitamin C (IVC) protocol for adjunctive cancer care: IVC as a
chemotherapeutic and biological response modifying agent. In: “The orthomolecular
treatment of chronic disease”, edited by AW Saul, Basic Health Publications, Inc. 2014,
750-766.
6. Du J, Cullena JJ, Buettnera GR. Ascorbic acid: Chemistry, biology and the treatment of
cancer. Biochim Biophys Acta. 2012; 1826:443-57.
7. Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients’ health-related
Quality of life after high dose vitamin C administration. J Korean Med Sci. 2007;
22(1):7–11.
8. Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety,
tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients
with advanced cancer. Cancer Chemother Pharmacol. 2013; 72(1):139–46.
9. Vollbracht C, Schneider B, Leendert V, Weiss G, Auerbach L, Beuth J. Intravenous
Vitamin C administration improves quality of life in breast cancer patients during
chemo-/radio therapy and after care: results of a retrospective,multicentre,
epidemiological cohort study in Germany. In vivo. 2011; 25(6):983–990
Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral
ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of
chemotherapy. Sci Transl Med. 2014; 6(222): 222ra18.
11. Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, et al.
Pharmacological ascorbate with gemcitabine for the control of metastatic and nodepositive
pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer
Chemother Pharmacol. 2013; 71(3):765–75.
12. Mayland CR, Bennett MI, Allan K. Vitamin C deficiency in cancer patients. Palliat Med
2005; 19:17–20.
13. Mikirova NA, Casciari JJ and Riordan NH. Ascorbate inhibition of angiogenesis in
aortic rings ex vivo and subcutaneous Matrigel plugs in vivo. Journal of Angiogenesis
Research. 2010; 2:2.
14. Mikirova N, Thomas E Ichim, Neil H Riordan. Anti-angiogenic effect of high doses of
ascorbic acid. Journal of translational medicine, 2008, 6:50
15. Mikirova N, Casciari J, Taylor P, Rogers A. Effect of high-dose intravenous vitamin
C on inflammation in cancer patients. Journal of Translational Medicine. 2012; 10:189.
16. Leibovitz B, Siegel BV. Ascorbic acid, neutrophil function, and the immune response.
International Journal for Vitamin and Nutrition Research, 1978; 48: 159–164.
17. Casciari, J, Riordan, N, Jackson J. Riordan H. 2001. Cytotoxicity of ascorbate, lipoic
acid, and other antioxidants in hollow fibre in vitro tumours. Br. J. Cancer. 2001; 84:
1544–50.
18. Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Beutner GR, Shacter E,
Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells:
action as a pro-drug to deliver hydrogen peroxide to tissues. PNAS USA, 2005; 205:
13604–13609.
19. Jihye Yun, Edouard Mullarky, Changyuan Lu, Kaitlyn N. Bosch, Adam Kavalier,Keith
Rivera, Jatin Roper et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal
cancer cells by targeting GAPDH. Science. 2015; 350: 1391-1396.
20. Geesin JC, Darr D, Kaufman R, Murad S, Pinnell SR. Ascorbic acid specifically
increases type I and type III procollagen messenger RNA levels in human skin
fibroblasts. J Investig Dermatol. 1988; 90: 420–424.
21. Miles SL, Fischer AP, Joshi SJ, Niles RM. Ascorbic acid and ascorbate-2-phosphate
decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer.
2015; 15:867
22. Karin M, Cao Y, Greten FR, Li ZW. NF-κB in cancer: from innocent bystander to major
culprit. Nature reviews. Cancer. 2002; 2: 301-310.
23. Cárcamo JM, Pedraza A, Bórquez-Ojeda O, Zhang B, Sanchez R, Golde DW. Vitamin
C is a kinase inhibitor: dehydroascorbic acid inhibits IkBα Kinaseβ. Molecular and
Cellular Biology. 2004; 6645–6652.
24. Bowie AG, O’Neill LA. Vitamin C inhibits NF-B activation by TNF via the activation
of p38 mitogen-activated protein kinase. J. Immunol. 2000; 165:7180-7188.
25. Pathi SS, Lei P, Sreevalsan S, Chadalapaka G, Jutooru I, et al. Pharmacologic doses of
ascorbic acid repress specificity protein (Sp) transcription factors and Sp-regulated
genes in colon cancer cells. Nutr Cancer. 2011; 63: 1133-1142.
26. Kim, JE, Jin DH, Lee SD, Hong SW, Shin JS, Lee KS, Jung DJ, Kang JS, Lee WJ.
Vitamin C inhibits p53-induced replicative senescence through suppression of ROS
production and p38 MAPK activity. Int. J. Mol. Med. 2008; 22: 651-655.
27. Battisti V, Maders LD, Bagatini MD, Reetz LG, Chiesa J, Battisti IE, Gonçalves JF,
Duarte MM, Schetinger MR, Morsch VM. Oxidative stress and antioxidant status in
prostate cancer patients: relation to Gleason score, treatment and bone metastasis.
Biomedicine & Pharmacotherapy. 2011; 65 (7):516-524.
28. Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold
and tumor evolution. Curr Opin Cell Biol. 2010; 22: 697–706.
29. Liotta LA. Tumor invasion and metastases-role of the extracellular matrix: Rhoads
Memorial Award lecture. Cancer Res. 1986; 46:1–7.
30. Mikirova N, Riordan N, Casciari J. Modulation of cytokines in cancer patients by
intravenous ascorbate therapy. Med Sci Monit, 2016; 22: 14-25.
31. Chen P, Yu J, Chalmers B, Drisko J, Yang J, Li B, Chen Q. Pharmacological ascorbate
induces cytotoxicity in prostate cancer cells through ATP depletion and induction of
autophagy. Anti-Cancer Drugs. 2012; 23 (4): 437-44.
32. Maramag C, Menon M, Balaji KC, Reddy PG, Laxmanan S. Effect of vitamin C on
prostate cancer cells in vitro: effect on cell number, viability, and DNA synthesis. The
Prostate [Prostate] 1997; 32 (3): 188-195.
33. Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. In vivo antitumor
effect of ascorbic acid, lysine, proline and green tea extract on human prostate cancer
PC-3 xenografts in nude mice: evaluation of tumor growth and immunohistochemistry.
In vivo. 2005; 19: 179-184.
34. Pollard H, Levine M, Eidelman O, Pollard M. Pharmacological ascorbic acid suppresses
syngenic tumor growth and metastases in hormone-refractory prostate cancer. In Vivo,
2010; 2012: 249–55.
35. Tareen B, Summers JL, Jamison JM, Neal DR, McGuire K, Gerson L, Diokno A. A 12
week, open label, phase I/IIa study using apatone for the treatment of prostate cancer
patients who have failed standard therapy. International Journal Of Medical Sciences.
2008; 5 (2): 62-7.
36. Stamey TA, Kabalin JN. Prostate specific antigen in the diagnosis and treatment of
adenocarcinoma of the prostate. I. Untreated patients. J. Urol. 1989; 141: 1070–5.
37. Partin AW, Carter HB, Chan DW, Epstein JI, Oesterling JE, Rock RC, Weber JP, Walsh
PC. Prostate specific antigen in the staging of localized prostate cancer: influence of
tumor differentiation, tumor volume, and benign hyperplasia. J. Urol. 1990; 143: 747–52.
38. Wolff JM, Bares R, Jung PK, Buell U, Jakse G. Prostate-specific antigen as a marker of
bone metastasis in patients with prostate cancer. Urol. Int. 1996; 56: 169–73.
39. Lieberherr M, Vrevan J, Vaes G. The acid and alkaline phosphatases, inorganic
pyrophosphatases, and phosphoprotein phosphatase of bone. Biochem. Biophys. Acta.
1973; 293: 160–169.
40. Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ. Serum alkaline phosphatase
changes predict survival independent of PSA changes in men with castration-resistant
prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012, 28(25):
e441-2.
41. Riordan NH, Riordan HD, Casciari JP. Clinical and experimental experiences with
Intravenous Vitamin C. J of Orthomolecular Medicine 2000; 15(4): 201-213.
42. Mikirova N, Casciari J, Riordan N, Hunninghake R. Clinical experience with
intravenous administration of ascorbic acid: achievable levels in blood for different
states of inflammation and disease in cancer patients. Journal: Journal of Translational
Medicine 2013, 11:191.
43. Teeter AE, Presti JC Jr, Aronson WJ, Terris MK, Kane CJ et al. Does PSADT after
radical prostatectomy correlate with overall survival?—a report from the SEARCH
database group. Urology. 2011; 77: 149–53.
44. Smaletz O, Scher HI, Small EJ, Verbel DA, McMillan A et al. Nomogram for overall
survival of patients with progressive metastatic prostate cancer after castration. J Clin
Oncol. 2002; 20: 3972–82.
45. Lorente JA, Morote J, Raventos C, Encabo G, Valenzuela H. Clinical efficacy of bone
alkaline phosphatase and prostate specific antigen in the diagnosis of bone metastasis in
prostate cancer. J Urol. 1996; 155: 1348–51
46. Ramankulov A, Lein M, Kristiansen G, Loening SA, Jung K. Plasma osteopontin in
comparison with bone markers as indicator of bone metastasis and survival outcome in
patients with prostate cancer. Prostate. 2007; 67: 330–40.
47. Robinson D, Sandblom G, Johansson R et al. Prediction of survival of metastatic
prostate cancer based on early serial measurements of prostate specific antigen and
alkaline phosphatase. J Urol. 2008; 179: 117–22.
48. Orwoll E, Teglbjærg CS, Langdahl BL et al. A randomized, placebo-controlled study of
the effects of denosumab for the treatment of men with low bone mineral density.
Journal of Clinical Endocrinology and Metabolism, 2012; 97: 3161– 3169.
49. Gartrell BA, Coleman RE, Fizazi K et al. Toxicities following treatment with
bisphosphonates and receptor activator of nuclear factor-kappa B ligand inhibitors in
patients with advanced prostate cancer. European Urology. 2014; 65(2): 278-86.
50. Takamizawa S, Maehata Y, Imai K, Senoo H, Sato S, Hata R. Effects of ascorbic acid
and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation
and differentiation of human osteoblastlike cells. Cell Biol Int. 2004; 28:255–265.





