The Levels of Ascorbic Acid in Blood and Mononuclear Blood Cells After Oral Liposome-Encapsulated and Oral Non-Encapsulated Vitamin C Supplementation, Taken Without and with IV Hydrocortisone
Introduction
The goal of this study was to compare concentrations of ascorbate (vitamin C) in plasma and white blood cells (WBCs) after oral administration of 1) liposomal vitamin C versus 2) traditional vitamin C in the form of powder and to analyze the effect of hydrocortisone on the improvement of ascorbate intake by cells.
Vitamin C functions as an effective antioxidant and has a major role in immunity by protecting immune cells against oxidative stress generated during infections (Carr et al., 2017). Humans do not have gulonolactone oxidase, which is the final enzyme in a sequence of enzymes in the liver required for vitamin C biosynthesis from glucose and must obtain vitamin C from the diet. Both plasma and white blood cell vitamin C concentrations can be increased significantly by dietary sources of vitamin C and especially by intake of vitamin C supplements.
Vitamin C levels in white blood cells are higher than in plasma, which may indicate functional roles of the vitamin in these immune system cells. In particular, leukocytes have been shown to have higher levels of vitamin C than are found in the plasma (Loh et al., 1971). These cells are typically the first immune cells to appear at a new site of inflammation and infection. Vitamin C has been shown to affect the functions of phagocytes, production of interferon, replication of viruses, maturation of T-lymphocytes, and other functions of the immune cells in laboratory studies (Carr et al., 2017).
Several significant findings of the importance of vitamin C on immune function are listed below (Levy, 2012):
- Vitamin C enhances antibody production (Feigen et al., 1982; Yamamoto et al., 1993; Azad et al., 2007);
- Vitamin C improves the function of macrophages and phagocytic white blood cells (Mohammed et al., 2014; Bozonet et al., 2015);
- Vitamin C increases interferon production (Karpinska et al., 1982; Kim et al., 2016);
- Vitamin C increases the proliferation of normal T-lymphocytes, while suppressing the proliferation of malignant T-lymphocytes (Huijskens et al., 2014; Molina et al., 2014; Uchio et al., 2015; Gao et al., 2017; Harakeh et al., 2017; Pangrazzi et al., 2017);
- Vitamin C promotes the maturation of T-cells (Manning et al., 2013);
- Vitamin C promotes the proliferation of B-lymphocytes (Schwager and Schulze, 1997);
- Vitamin C induces and enhances natural killer cell activity (Heuser and Vojdani, 1997).
The second goal of the project was to demonstrate that IV hydrocortisone when administered at the time of supplementation by the oral form of vitamin C can increase the transport of ascorbate into white blood cells, resulting in the higher intracellular ascorbic acid concentrations. Animal studies, along with human clinical studies, support the concept that vitamin C and hydrocortisone appear to be designed by nature to naturally interact with each other to optimize the antioxidant impact in diseased and infected tissues, and to directly promote the recovery of their normal functions while accelerating their healing. Hydrocortisone has also been studied extensively with regard to its effects and impacts on various pathophysiological aspects of sepsis, as well as the infectious conditions leading to sepsis (Carr, 2018; Wilson, 2009; Marik, 2017). In a cell study, corticosteroids (dexamethasone) increased the expression of the vitamin C transporter SVCT2, enabling a significant increase in the uptake of vitamin C into those cells (Fujita et al., 2001).
The evaluation of the concentrations of vitamin C in serum and blood cells after intake of the oral liposomal and non-encapsulated vitamin C supplementation without and with IV hydrocortisone may improve recommendations of the dosages and frequencies of the ascorbate supplementation.
Study Design
Five subjects participated in the study. The investigation was carried out following the rules of the Declaration of Helsinki of 1975 (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/), revised in 2008. Written informed consent was provided by all patients. The study was approved by the Institutional Review Board of the Riordan Clinic (Wichita, KS). The enrolled subjects were advised not take vitamin C five days before intervention and for four weeks during the study. The subjects also advised to refrain from anti-inflammatory medicines and multivitamins that contain vitamin C.
The type of supplementations and blood drawing schedule are presented in Table 1. The data in the table show the type of supplementation that each subject had once at the beginning of the week (on Monday), and the intervals of the day when blood samples were taken.
Table 1.
For this research study, the monitoring/follow-up procedures included: one heparin tube of blood drawn before, and 2h, 4h, and 6h after intervention and four measurements of ascorbic acid in plasma and in mononuclear white blood cells.
The intervention protocol consisted of:
- Initial one week wash out period (control baseline);
- The first week: single 5 g dose of sodium ascorbate powder (NOW Foods) and tracking Q2H ascorbate levels in blood plasma and cells before and for 6 hours after dose; six days wash out period;
- The second week: single 5 g ascorbate dose in liposomal formulation (LivOn Lab) and tracking Q2H ascorbate levels in blood plasma and cells before and for six hours after dose; six days wash-out period;
- The third week: infusion of 50 mg of hydrocortisone (Solu-Cortef, Pfizer) and intake of 5 grams ascorbate as powder with tracking Q2H the concentrations of ascorbate in plasma and cells before and for 6 hours after dose; 6 days wash-out period;
- The forth week: infusion of 50 mg of hydrocortisone and intake of 5 grams liposomal ascorbate with tracking Q2H the concentrations of ascorbate in plasma and cells before and for 6 hours after dose.
The concentrations of ascorbate were compared between the same subjects plus averaged for all subjects for each intervention.
Measurements
Blood samples collected at baseline (0 h) and 2, 4 and 6 h post-dose were analyzed for the pharmacokinetics of plasma and leukocyte vitamin C. Blood was collected in a sodium heparin tube, kept on rocker to prevent coagulation and analyzed as soon as possible. One ml of blood was used for the plasma ascorbate measurements and the rest of the blood for the separation of white blood cells (WBCs).
The concentration of ascorbate in plasma and inside the cells was measured by the test based on research study of Vislisel et al., 2007 and Ascorbate Assay Kit (Cayman Chemical). This method of analysis of total ascorbate and oxidized ascorbate is based on the well-known condensation reaction of dehydroascorbic (DHA) with o-phenylenediamine (OPDA) to form a highly colored product that can be detected by fluorescence.
The level of ascorbate and DHA was analyzed by fluorescence (Fluorometer-3, Jobin Yvon, SPEX) with an excitation wavelength 350 nm and an emission wavelengths between 420 -430 nm.
For measurements of the ascorbate in WBCs, the buffy coat layer was collected by Ficoll-Paque Plus. Cells were washed twice with PBS, and the cells counts were performed by standard lab procedure (by hemocytometer or flow cytometer). Cell pellets were frozen at -80°C to rupture the membranes. After freezing (30 min at −80°C), cell pellets are thawed and extracted with 200 μL MeOH/H2O/DTPA (75/22.5/ 2.5, v/v/v), incubated on ice for 10 min, and then centrifuged at 12,000 g for 5 min. Supernatant was stored at -80°C until analysis.
For plasma ascorbate measurements, blood (1 ml) was centrifuged at 2000g for 15 min at 4 C. The top yellow plasma layer was collected. 800 ul of (90:7.5:2.5) methanol/water/DTPA were added to 200 ul of plasma, vortexed and incubated on ice10 min. After 10 min, tubes were centrifuged at 4C and 12000g for 10 min. The supernatant was collected and stored on ice.
Results of the Study
Plasma Vitamin C After Oral Liposome-Encapsulated and Non-Encapsulated Ascorbate Supplementation
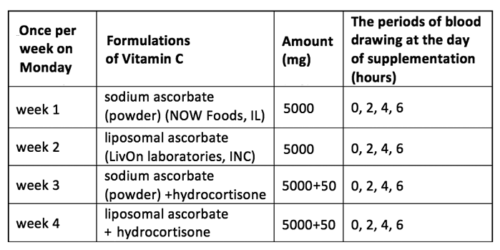
The changes of ascorbate concentrations in plasma of each subject after oral supplementation of 5 grams of non-encapsulated ascorbate are demonstrated in Figure 1. The maximum concentrations of ascorbate in plasma were achieved 2 h after intake of ascorbate and then declined in intensity on average 25% during next 4 hours.
As all subjects had different initial concentrations of AA in plasma, we correlated the increase in plasma ascorbate with initial measured levels. A linear relation between the maximum concentrations in plasma and initial levels of ascorbate was observed (r=0.9).
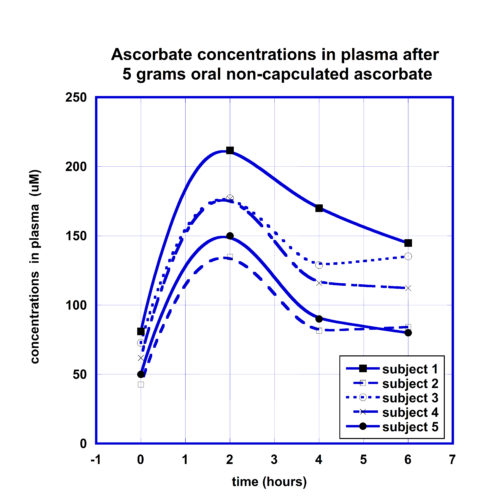
For the comparison of the kinetic curves of the plasma ascorbate concentrations after the same dose liposomal and non-encapsulated ascorbate supplementations, the ratios of ascorbate concentrations in plasma to initial levels were calculated for each participant at each time point, values were averaged and presented for different time points in Figure 2. Data in Figure 2 show the means and standard deviations of ascorbate in plasma for two forms of supplementations.
According to these data, in plasma the maximum concentration of ascorbate encapsulated in the liposomal carrier was reached 4 hours after supplementation in comparison with maximum at 2 hours for non-encapsulated form of ascorbate. After liposomal ascorbate intake, in average the concentration of ascorbate in plasma was increased on 110% after 2 hours, reached maximum levels of 160% after 4 hours and decreased to the level 125% after 6 hours. For the non-encapsulated form of ascorbate the maximum level of 170% increase was reached after 2 hours and then decreased to 90% and 80% at the 4 and 6 hours post-dose measurements.
The area under the curve (AUC) calculated by trapezoidal summation equals 799 uMh for liposomal ascorbate and 773 uMh for non-encapsulated ascorbate. In addition, the kinetic curves demonstrate longer retention of the ascorbate in the body after the liposomal supplement in comparison with the supplement in non-encapsulated form. In average, the ascorbate concentration remained in plasma at the level two times higher than initial during 3 hours for non-encapsulated ascorbate and longer than 4.5 hours for liposomal ascorbate.
In summary, no significant difference was noted in the maximum concentrations of ascorbate in plasma between liposome-encapsulated and non-encapsulated ascorbate, but a difference was found in the retention time of the ascorbate in blood with liposomal formulation retention longer than the retention of non-encapsulated ascorbate.
Ascorbate Concentrations in White Blood Cells After Liposome-Encapsulated and Non-Encapsulated Ascorbate Supplementation
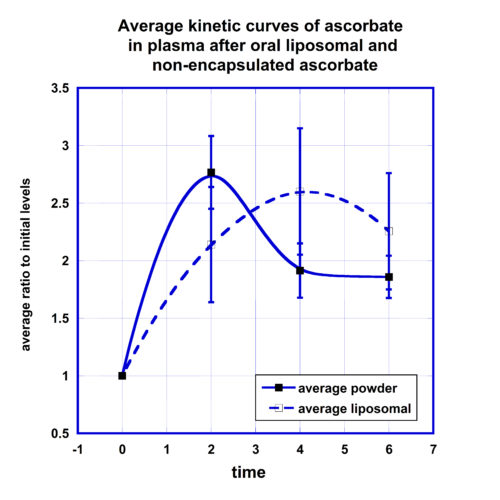
Comparisons of the concentrations of ascorbate in WBCs for two forms of supplementations are presented in Figure 3. Data in graph show the average concentrations normalized on the initial levels of intracellular ascorbate with standard deviations.
After liposomal supplementation the level of ascorbate in cells was increased by 30% two hours after supplementation, reached maximum 50% increase after 4 hours, and sustained retention of 30% increase after 6 hours.
For non-encapsulated ascorbate the baseline level did not change two hours post-dose, then increased by 40% after 4 hours and decreased to 15% above the average normalized initial concentration at the 6-hour time point. The data in Figure 3 suggest faster intake of liposomal ascorbate by cells. The comparison of the areas under the curve for concentrations that were higher than initial levels showed that AUC was 50% larger for liposomal formulation in comparison with non-encapsulated ascorbate.
Another interesting observation was that of the amount of ascorbate incorporated in the cells depended on the initial level of ascorbate in WBCs. For the lower initial intracellular level of ascorbate 50 nm/108cells the increase of the ascorbate concentration in cells was higher (180%) than for higher initial concentrations 100 nm/108 cells (80%).
The Effect of Hydrocortisone on Ascorbate Intake in Cells
To evaluate the effect of hydrocortisone on the intake of ascorbate by cells, we compared the kinetic curves of the intracellular ascorbate concentrations after oral liposome-encapsulated vitamin C supplementation and the same dosage of the liposomal supplementation followed by 50 mg IV hydrocortisone. The data are presented in Figure 4.
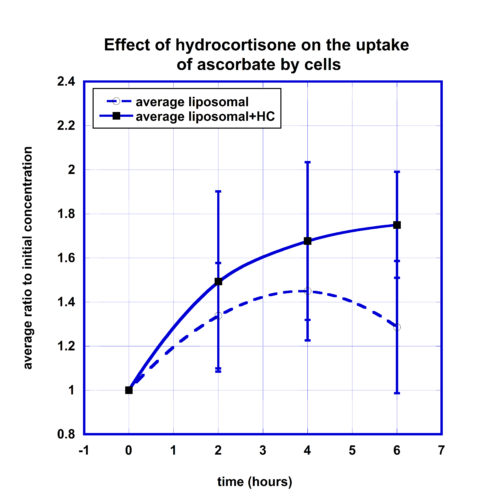
According to these data, the intracellular concentration after liposomal ascorbate supplementation increased from baseline level to maximum level 45% four hours post-dose and then decreased to 30% at 6 hour time point.
The effect of hydrocortisone resulted in more favorable percentage of change at all time points with the final increase to 75% at 6 hours post-dose. The difference at the 6 hour time point was statistically significant (p-value<0.05). The area under normalized concentration-time curve for values higher than initial levels was on 60% larger for liposomal supplementation taken after IV hydrocortisone.
A similar difference was found for non-encapsulated ascorbate supplementation taken with and without IV hydrocortisone injections (Figure 5).
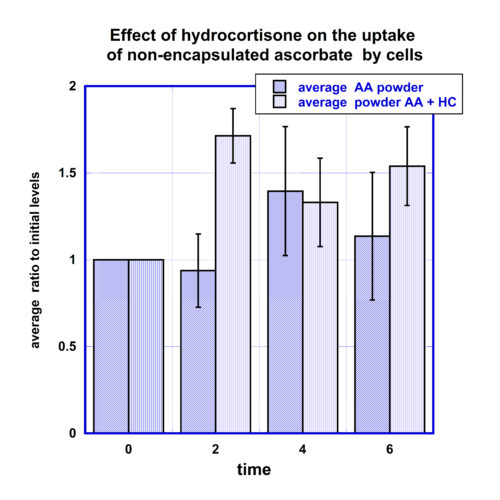
The intracellular ascorbate concentrations after intake of ascorbate in the form of oral non-encapsulated ascorbate after IV hydrocortisone injection showed an increase above the baseline intracellular concentrations by 70% at the 2 hour time point, and 30% and 50% for next measurements at 4 and 6 hours. After an intake of 5 grams of oral non-encapsulated ascorbate without hydrocortisone, the average maximum increase of intracellular concentrations was 40% at four hours post intervention.
Summary
- Comparisons of the liposome-encapsulated and non-encapsulated (powder) ascorbate supplementations demonstrated that liposomal ascorbate intake results in longer retention of ascorbate in blood. Plasma concentrations of ascorbate remained at the level of 100% increase during 3 hours for non-encapsulated ascorbate and longer than 4.5 hours for liposomal ascorbate. The maximum percentage of ascorbate increase was the same for both formulations and reached 150%-170%.
- The concentrations of ascorbate in white blood cells were changed after intake of ascorbate in the form of non-encapsulated and encapsulated liposomal ascorbate supplementations. The average maximum increase in the concentrations of ascorbate in cells was the same for both formulations (in range 40%÷50%). The data show that liposomal ascorbate resulted in faster intake by cells. The comparison of the areas under the curve for concentrations that were higher than initial levels showed that AUC was on 50% larger for liposomal formulation in comparison with non-encapsulated ascorbate.
- The data support our hypothesis that hydrocortisone can have effect on the uptake of ascorbate by cells, when it is given as an adjuvant to ascorbate supplementation. The injection of hydrocortisone before intake of supplements resulted in more favorable percentage of intracellular ascorbate intake.
- The weakness of the study is the low number of the participants and the measurements of ascorbate concentrations in plasma and cells by a method based on the condensation reaction of dehydroascorbic with formation of colored product that was detected by fluorescence.
Authors’ Contributions
Nina Mikirova measured analyzed and interpreted data. Tom Levy and Ronald Hunninghake drafted the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
Azad I, J Dayal, M Poornima, S Ali (2007) Supra dietary levels of vitamins C and E enhance antibody production and immune memory in juvenile milkfish, Chanos chanos (Forsskal) to formalin-killed Vibrio vulnificus. Fish & Shellfish Immunology 23:154-163. PMID: 17208456
Bozonet S, Carr A, Pullar J, Vissers M (2015) Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 7:2574-2588. PMID: 25912037
Carr A, Maggini S. (2017) Vitamin C and Immune Function. Nutrients 9, 1211.
Carr A (2018) Can a simple chemical help to both prevent and treat sepsis. Critical Care 22:247
Feigen G, B Smith, C Dix et al. (1982) Enhancement of antibody production and protection against systemic anaphylaxis by large doses of vitamin C. Research Communications in Chemical Pathology and Pharmacology 38:313-333. PMID: 7163630
Fujita I, J Hirano, N Itoh et al. (2001) Dexamethasone induces sodium-dependent vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. The British Journal of Nutrition 86:145-149. PMID: 11502226
Gao Y, B Lu, J Zhai et al. (2017) The parenteral vitamin C improves sepsis and sepsis-induced multiple organ dysfunction syndrome via preventing cellular immunosuppression. Mediators of Inflammation 2017:4024672. PMID: 28210072
Harakeh S, J Khalife, E Baydoun et al. (2017) Effects of ascorbic acid on Tax, NF-kB and MMP-9 in human T-cell lymphotropic virus type 1 positive malignant T-lymphocytes. Anti-Cancer Agents in Medicinal Chemistry Jul 25 [Epub ahead of print]. PMID: 28745235
Heuser G, A Vojdani (1997) Enhancement of natural killer cell activity and T and B cell function by buffered vitamin C in patients exposed to toxic chemicals: the role of protein kinase-C. Immunopharmacology and Immunotoxicology 19:291-312. PMID: 9248859
Huijskens M, M Walczak, N Koller et al. (2014) Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. Journal of Leukocyte Biology 96:1165-1175. PMID: 25157026
Karpinska T, Z Kawecki, M Kandefer-Szerszen (1982) The influence of ultraviolet irradiation, L-ascorbic acid and calcium chloride on the induction of interferon in human embryo fibroblasts. Archivum Immunologiae et Therapiae Experimentalis 30:33-37. PMID: 7149924
Kim H, M Jang, Y Kim et al., (2016) Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. The Journal of Pharmacy and Pharmacology 68:406-420. PMID: 26898166
Levy TE (2012) Primal Panacea. MedFox Publishing, LLC.
Loh HS, Wilson CWM (1971) Relationship Between Leucocyte and Plasma Ascorbic Acid Concentrations. British medical journal 3, 733-735.
Manning J, B Mitchell, D Appadurai et al. (2013) Vitamin C promotes maturation of T-cells. Antioxidants & Redox Signaling 19:2054-2067. PMID: 23249337
Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J (2017) Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock A Retrospective Before-After Study Chest, 152:690.
Mohammed B, B Fisher, Q Huynh et al. (2014) Resolution of sterile inflammation: role for vitamin C. Mediators of Inflammation 2014:173403. PMID: 25294953
Molina N, A Morandi, A Bolin, R Otton (2014) Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. International Immunopharmacology 22:41-50. PMID: 24975831
Pangrazzi L, A Meryk, E Naismith et al. (2017) “Inflamm-aging” influences immune cell survival factors in human bone marrow. European Journal of Immunology 47:481-492. PMID: 27995612
Schwager J, J Schulze (1997) Influence of ascorbic acid on the response to mitogens and interleukin production of porcine lymphocytes. International Journal for Vitamin and Nutrition Research 9119607
Uchio R, Y Hirose, S Murosaki et al. (2015) High dietary intake of vitamin C suppresses age-related thymic atrophy and contributes to the maintenance of immune cells in vitamin C-deficient senescence marker protein-30 knockout mice. The British Journal of Nutrition 113:603-609. PMID: 25608928
Yamamoto I, M Tanaka, N Muto (1993) Enhancement of in vitro antibody production of murine splenocytes by ascorbic acid 2-O-alpha-glucoside. International Journal of Immunopharmacology 15:319-325. PMID: 8505144
Vislisel JM, Schafer FQ, Buettner GR (2007) A simple and sensitive assay for ascorbate using a plate reader. Anal Biochem 365: 31–39.
Wilson JX (2009) Mechanism of action of vitamin C in sepsis: Ascorbate modulates redox signaling in endothelium. Biofactors 35: 5–13.





