Clinical Test of Pyrroles: Usefulness & Association with Other Biochemical Markers
Clinical Test of Pyrroles: Usefulness and Association with Other
Biochemical Markers
Nina Mikirova
Riordan Clinic, Wichita, USA
Corresponding author: Nina Mikirova, Riordan Clinic, 3100, N Hillside, Wichita, KS, USA, Tel: 3169274753,
E-mail: nmikirova@riordanclinic.org
Abstract
Background: Psychiatrists started using urine pyrroles
(hydroxyhemopyrrolin-2-one, HPL) to diagnose mental disorders
many years ago. The biological origins of HPL are not known,
nor are the causes of elevated urinary pyrrole excretion well
understood.
Methods: In the present study we analyzed the level of pyrroles in
148 patients with schizophrenia, 135 patients with bipolar disorder,
97 patients with depression, and 119 patients with ADHD and
compared these data with the results of pyrrole tests for patients
with non-mental conditions and healthy volunteers.
Results: According to our data, urinary pyrrole concentrations
tended to be high in patients with mental illnesses, but elevated
level of pyrroles was not specific for only these patients. We
found evidence of an allergy related component in the fact that
elevated pyrrole levels were significantly more prevalent in subjects
with elevated histamine values. A role of intestinal bacteria, or
imbalances in intestinal bacterial metabolism, was also suggested
based on the found relationship between elevated pyrrole levels
and elevations in indicans and urobilinogens. In addition, our data
demonstrated that subjects with severely elevated pyrrole levels
were deficient in nutrients such as zinc, vitamin B3, and vitamin C.
Conclusion: Thus, pyrrole excretion seems to be a component of
illness in general and not strictly mental illness.
Keywords
Mental illness, Pyrroles, Indicans, Urobilinogens, Histamine,
Nutrient deficiency
Introduction
Interest in pyrroles as markers of mental illness started Dr. Abram
Hoffer’s discovery that “Mauve Factor”, a pyrrole named for its
lavender appearance in urine chromatograms stained with Ehrlich’s
reagent [1], was prominent in urine samples from schizophrenics
[2,3]. After considerable effort [4-10], Mauve Factor was determined
to be the hemopyrrole derivative hydroxyhemopyrrolin-2-one
(HPL). Hoffer claimed that HPL tended to decrease when a patient
recovered from illness, and increased when illness reappeared;
moreover, treatments with vitamin B6 and zinc were reported to
decrease HPL levels and were associated with patient recovery [11]. Some psychiatrists, particularly those with interests in
orthomolecular medicine, have used HPL as a clinical tool for
diagnosing and following the progression or remission of mental
illness [11-17]. Data from these studies suggest that roughly onethird of schizophrenia patients tested had elevated pyrroles, but high
urine HPL levels were not limited to schizophrenia, as a variety of
conditions and stresses are associated with urine pyrrole excretion.
The biological origins of HPL are not known, nor are the
causes of elevated urinary pyrrole excretion understood. Proposed
mechanisms for HPL formation and accumulation in the body
include intake from dietary sources, heme breakdown, or altered
heme biosynthesis, the latter perhaps occurring with the aid of gut
flora [18]. Irvine has proposed that HPL is a metabolite of heme
synthesis intermediates porphobilinogen and prophyrins, as these
are structurally very similar to HPL [19]. Urine HPL levels can be
quantified using a colorimetric assay, provided precautions are taken
to keep it stable prior to and during the assay. Increases in pyrrole
levels and excretion may occur as a result of stress-induced changes
in intestinal permeability, which in turn leads to increased pyrrole
absorption. To the extent that pyrrole excretion may be an indicator
of heme breakdown due to emotional stress, oxidative stress, or
nutrient deprivation, a study of the correlation between urinary
pyrroles and nutrient levels should be of interest [18,20-22].
At the Riordan Clinic, urinary pyrrole measurements have
been part of the protocol for diagnosing mental illnesses and other
disorders for decades. Access to the clinic’s database has enabled us
to examine pyrrole levels in patients with a variety of illnesses and to
see how they correlate with the concentrations of other key nutrients
or metabolites. The present manuscript describes our analysis and
evaluation of these data, and provides some assessment of the
potential value of monitoring pyrrole levels in mentally ill patients.
Materials and Methods
All laboratory tests were conducted by the Riordan Clinic
Bio-Center Laboratory (Wichita, KS), a licensed and certified
medical laboratory that offers over one-hundred laboratory
tests (http://www.riordanclinic.org/laboratory/catalog.pdf).
Handling of laboratory data by our institute is done in full compliance
with HIPAA regulations. Assay methods for the various vitamins,
minerals, and lipids for which data are presented here were conducted
measurement of urine HPL, urine was stabilized with ascorbic
acid (8mL urine added to 500mg of ascorbic acid) and frozen to
ensure HPL stability. Pyrroles were them extracted from urine with
chloroform and reacted with Ehrlich’s aced aldehyde reagent (0.5g
of p-dimethylaminobenzaldehyde, 2.5 ml sulfuric acid in 50ml of
methanol). This preparation yields a chromophore with an absorption
maximum of 540 nm, which is related to HPL concentration using a
standard curve. The comparison of the colorimetric assay for HPL
with highly sensitive and specific HPL assay, which utilizes highpressure liquid chromatography mass spectroscopy (HPLC/MS),
showed the high level of correlation between these two assays (r=0.9;
P<.0001) [18].
Data from the Bio-Center Laboratory were obtained using the
LabNet program (Henry Schein, Melville NJ). Statistical analyses
were carried out using the Excel spreadsheet program and graphs,
with regression data fits where appropriate, were constructed using
the Kalaidagraph program (Synergy Software, Reading PA).
Results
We analyzed urine pyrrole data in 119 patients with ADHD, 148
patients with schizophrenia, 135 patients with bipolar disorder and
97 patients with depression. We first examined parameters that are
key precursors for neurotransmitters (amino acids) or have been
cited as being potentially relevant to mental illness, such as toxic
metals, essential minerals, and fatty acids. The average values of these
tests are shown in Table 1.
Tests for mental illness groupings show elevated average values of
hair aluminum, lead (in patients with schizophrenia), iron (total and
hair), Arachidonic acid to EPA ratio, omega 3 to omega 6 fatty acid ratios,
and urine pyrroles. Deficiencies were found in the levels of essential
metals magnesium, zinc and copper (serum). Some deficiencies and
the metabolic imbalances in fatty acid, amino acid, mineral, and pyrrole
levels in ADHD patients were analyzed previously [19].
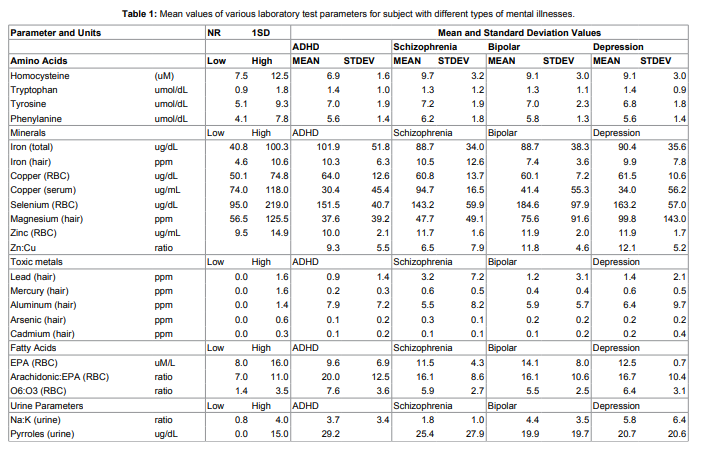
The percentage of patients with increased levels of pyrroles in
comparison with patients without specific diagnosis (134 healthy
subjects) is shown in Figure 1.
pyrroles higher than 20ug/dL was 48% for ADHD patients, 22%
for patients with schizophrenia, 30% for patients with bipolar and
depression and 26% for patients without specific diagnosis.
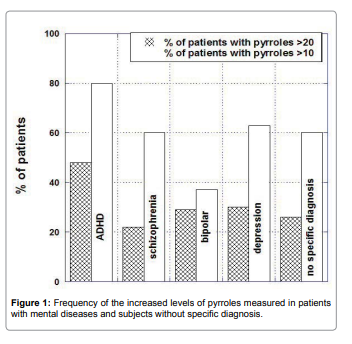
Pyrrole distributions in subjects with mental illnesses were
analyzed by illness category, along with the distribution that would
be expected if all subjects were in the normal range. There was skew
in the data indicating subjects with elevated pyrrole levels. It is
particularly acute in ADHD patients, of whom 48% had levels above
the normal range (Figure 2).
The mean of pyrrole values outside the normal range were 47ng/
dL for patients with schizophrenia, 43ng/dL for patients with bipolar
disorder, 39ng/dL for patients with depression and 44ng/dL for
patients with ADHD.
The highest level was measured for a 10-year old boy (481ug/
dL). A level of 192ug/dL was found in 5 year old girl and levels of
12 3ug/dL and 114ug/dL in two boys. The proportion of subjects
with pyrroles outside the normal range was similar to those seen in
patients with other illnesses (26 % of all subjects analyzed had pyrrole
levels above 20ug/dL), suggesting that pyrrole levels indicate illness in
general and not necessarily mental illness.
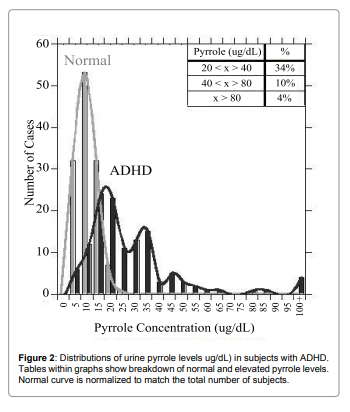
To see if pyrrole levels were related to allergic reactions, we
compared pyrrole levels with histamine and immunoglobulin levels.
For histamine, data are shown in Figure 3a and Figure 3b.
There was a statistically significant correlation (p<0.001) between
histamine levels and pyrrole levels, and the vast majority of patients
with elevated pyrrole levels showed elevated histamine levels also. For
instance, for subjects with histamine levels below 53ng/dL (the upper
limit of normal in our laboratory, only 16% of subjects had elevated
(>20ug/dL) pyrrole levels, with only 2% having pyrrole values above
40ug/dL and only 2% having values above 80ug/dL. In contrast,
26% of subjects with elevated histamine levels had elevated pyrrole
levels, with 9% showing values above 40ug/dL and 4% showing values
above 80ug/dL. All subjects with pyrrole levels above 100ug/dL had
elevated histamine levels. Similar trends were observed with the
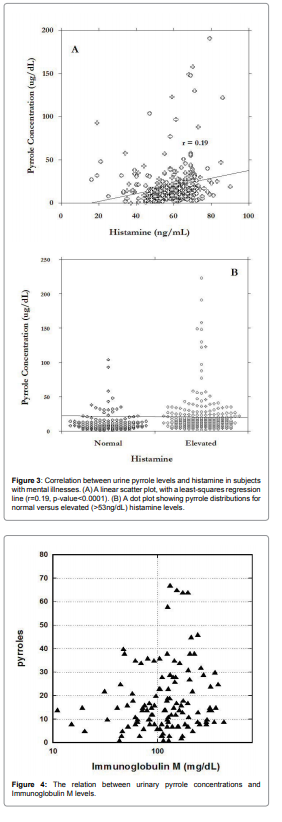
immunoglobulins IgM. Relation between IgM and pyrroles is shown
in Figure 4.
For abnormal levels of pyrroles (>2ug/dL), 75% of data were in
the range of IgM higher than 100mg/dL. Average level of pyrroles
was 30ng/dl ± 8ng/dl for IgM<100mg/dl and 46ng/dl ± 40ng/dl for
IgM>100mg/dL.
Elevated pyrroles are also thought to be related to intestinal
issues. The urine indican test is considered an indicator of intestinal
toxemia and overgrowth of anaerobic bacteria (indican is a product
of bacterial tryptophan digestion), while urobilinogens are products
of intestinal bacteria that can build up if the liver is overburdened.
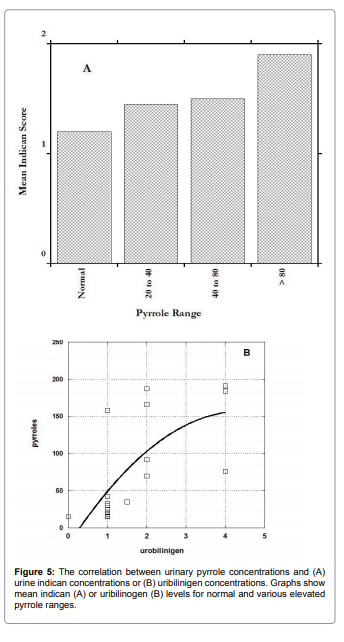
Figure 5 shows how these parameters vary depending on urinary
pyrrole levels. In both cases, elevated pyrroles are associated with
elevated levels, supporting the hypothesis that intestinal bacteria
overgrowth may be associated with excessive pyrrole excretion.
To determine if increased pyrroles secretion is accompanied by
nutritional deficiencies, we compared urinary pyrrole concentrations
with vitamins and minerals in blood for cases where both were
measured on the same visit. Vitamins are natural barriers against
infection and allergic reactions, as well as chemical balance of essential
minerals. Examples where a relationship was particularly noticeable
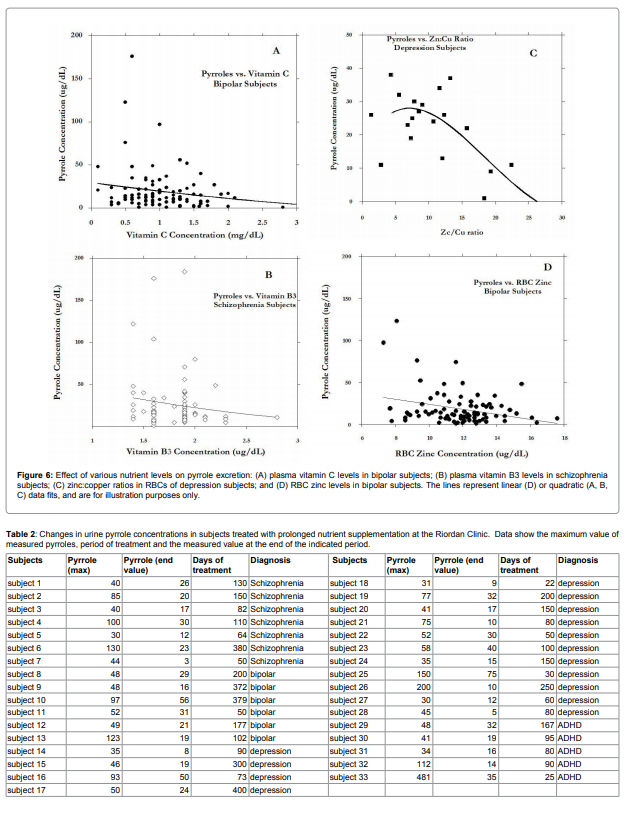
are shown in Figure 6.
Vitamin C, vitamin B3, red blood cell zinc, and zinc to copper
ratios were found in some mental illness groups to decrease with
increasing pyrrole levels. Examining Figure 6, it appears that subjects
with very high pyrrole levels (above 40ug/dL) tended toward the left
hand side of the horizontal axis, suggesting that data points with
very high pyrrole levels had low nutrient values. The potential link
between nutrient levels and pyrrole levels deserves further more
systematic study.
At the Riordan Clinic, patients with mental illness are sometimes
treated by attempting to restore proper mineral balances and correct
nutrient deficiencies. In particular, the integrative approach to
the treatment of elevated pyrroles includes: intravenous ascorbic
acid (5g-15g), Plex IV INF, vitamin B6 IV infusion, fortified flax,
super EPA, cod liver oil, zinc boost, lipoic acid, ProEFA, chelated
magnesium, DHEA and amino acids (cysteine, glutamine, tyrosine,
arginine). Other nutrients were added to assist in pyroluria include
niacinamide, pantothenic acid, manganese, Evening Primrose Oil
and digestive enzymes. We examined the database for mental illness
patients who underwent this type of therapy (though precise protocol
details varied from subject to subject) and compared “initial” to
“final” pyrrole values. Results are shown in Table 2.
These data suggest that pyrrole levels decreased during treatment.
We have not yet conducted a controlled trial where a consistent
treatment is used and patient mental illness (symptom severity) is
also monitored over time.
Discussion
The Riordan Clinic has been measuring nutrients, minerals,
toxins and urine pyrrole concentrations as a diagnostic tool for over
forty years. In examining laboratory parameters that are thought to
be important in maintaining proper neurological function (Table 1),
we found several potential trouble signs in patients with various types
of mental illnesses. In particular, the tests demonstrated the elevated
average values of aluminum, lead (in patients with schizophrenia),
iron, arachidonic acid to EPA ratio, omega 3 to omega 6 fatty acid
ratios, and urine pyrroles. Deficiencies were found in the levels
of essential metals magnesium, zinc and copper (serum). Urinary
pyrrole concentrations also tended to be high in these patients. We
investigated these elevated pyrroles further, finding that nearly half of
the patients diagnosed with ADHD had pyrrole concentrations above
the normal limit (20ug/dL) in urine. The proportion was roughly onethird in subject with depression, bipolar disorder, or schizophrenia.
Interestingly, that elevated level of pyrroles was not specific for only
patients with mental illnesses. When we analyzed patients without
mental diseases all together as a whole (with illnesses ranging from
cancer to arthritis, fibromyalgia, and chronic fatigue, among others)
and subjects without specific diagnosis, roughly a quarter of them
have elevated urinary pyrrole levels. Thus, pyrrole excretion seems
to be a component of illness in general and not strictly mental illness.
We are struck, though, by how high urine pyrroles can be in mentally
ill subjects, with roughly ten percent having values above 40ug/dL
and, in the case of schizophrenics and ADHD sufferers, nearly 5%
of subjects having values above 80ug/dL. We saw some patients with
levels over 200ug/dL.
Our examination of the Riordan Clinic database confirms some of
the conventional wisdom about pyrrole excretion. We find evidence
of an allergy component in the fact that elevated pyrrole levels were
significantly more prevalent in subjects with elevated histamine
values (Figure 3). Immunoglobulins also appear to correlate with
pyrroles (IgM vs pyrroles).
A role of intestinal bacteria, or imbalances in intestinal bacterial
metabolism, is also suggested based on the relationship between
elevated pyrrole levels and elevations in indicans and urobilinogens
(Figure 5). Indicans can be an indicator of protein digestion efficiency
[18]. Patients with high urine indicans can be expected to have issues
such as insufficient gastric hydrochloric acid, insufficient digestive
enzymes, adverse food reactions, infection, or bacterial overgrowth.
These problems are also consistent with elevated urobilinogens.
Elevated urine pyrroles, as they correlate with these other stress
factors, may simply be an indicator of metabolic stress in the body. It
also appears to be a general rule that subjects with severely elevated
pyrrole levels were deficient in nutrients such as nutrients zinc,
vitamin B3, vitamin C (Figure 6). The stress factors described above
(infection, toxicity, etc.) may be factors in causing nutrient deficiency,
or may be caused by nutrient deficiency. Vitamin C in particular is
important for innate and cell mediated immunity, as it protects
neutrophils from oxidative damage. The zinc to copper ratio is also an
indicator of oxidative stress. This redox variable is commonly outside
its normal range in mentally ill subjects, and appears to correlate in
some cases with pyrrole excretion.
At the Riordan Clinic, mentally ill subjects are sometimes
treated with supplements (including injections of B-vitamins, and
intravenous infusions of vitamin C) to replenish depleted nutrient
stores and provide for rejuvenation of the immune system. We were
thus interested in how these treatments affected pyrrole levels. While
we have not conducted a rigorous clinical study in this regard, we
were able to extract from the database a group of subjects who had
similar treatments and for whom pyrrole analyses before and after
extended treatment was available. The results, shown in Table 2, seem
to suggest that pyrrole concentrations are reduced after prolonged
supplementation therapy. This may be an interesting topic to explore
with more rigorous controlled studies.
In summary, our analysis of the Riordan Clinic patient database
suggests that pyroluria is relevant to a variety of stress and illness
conditions, and, to the extent that these stresses are relevant in mental
illness, is relevant to many mentally ill subjects. Abnormal pyrrole
excretion appears to be an indicator of oxidative stress, infection,
intoxication, or improper digestion. Moreover, our data suggest
that it may be possible to reduce pyroluria using a supplementation
treatment regimen such as that employed at the Riordan clinic.
References
1. Irvine DG (1961) Apparently non-indolic Ehrlich-positive substances related
to mental illnesses. J Neuropsychiatr 2: 292-305.
2. Hoffer A, Osmond H (1963) Malvaria: A new psychiatric disease. Acta
Psychiatr Scand 39: 335-366.
3. Hoffer A (1963) The presence of malvaria in some mentally retarded children.
Am J Ment Defic 67: 730-732.
4. Irvine DG, Bayne W, Miyashita H, Majer JR (1969) Identification of
kryptopyrrole in human urine and its relation to psychosis. Nature 224: 811-
813.
5. Irvine DG (1963) Mauve factor and 6-sulfatoxy skatole: two biochemical
abnormalities associated with specific measures of psychiatric disease. Clin
Chem 9: 444-445.
6. Irvine DG, Bayne W, Miyashita H (1973) The main form of naturally-occurring
kryptopyrrole: its 5-OH-2-lactam, a product of pyrrolooxygenase. Report
to the Psychiatric Research Meeting of the Saskatchewan Psychiatric
Association 46-67.
7. Irvine DG, Bayne W, Miyashita H, Majer JR (1969) Identification of
kryptopyrrole in human urine and its relation to psychosis. Nature 224: 811-
813.
8. Sohler A, Beck R, Noval JJ (1970) Mauve factor re-identified as 2,4-dimethyl3-ethylpyrrole and its sedative effect on the CNS. Nature 228: 1318-1320.
9. Irvine DG, Wetterberg L (1972) Kryptopyrrole-like substance in acute
intermittent porphyria. Lancet 2: 1201.
10. Irvine DG, Wilson DL (1976) Oxidized monopyrroles in porphyric disorders
and related conditions. In: Doss M Porphyrins in Human Diseases 217-224.
11. Hoffer A (1966) Malvaria, schizophrenia and the HOD test. Int J
Neuropsychiatry 2: 175-178.
12. Pfeiffer CC, Iliev V (1973) Pyroluria, urinary mauve factor causes double
deficiency of B6 and zinc in schizophrenics. Fed Am Soc Exp Biol 32: 276.
13. Pfeiffer CC, Sohler A, Jenney EH (1974) Treatment of pyroluric schizophrenia
(malvaria) with large doses of pyridoxine and a dietary supplement of zinc. J
Appl Nutr 26: 21-28.
14. Irvine DG (1974) Kryptopyrrole and other monopyrroles in molecular
neurobiology. Int Rev Neurobiol 16: 145-182.
15. Sohler A, Holsztynska MS, Pfeiffer CC (1974) A rapid screening test for
pyroluria; useful indistinguishing a schizophrenic population. J Orthomolec
Psychiatr 3: 273-279.
16. Jackson JA, Riordan HD, Neathery S (1990) Vitamin, blood lead, and urine
pyrrole levels in Down syndrome. Am Clin Lab: 1: 8-9.
17. Sohler A, Renz RH, Smith S, Kaufman J (1967) Significance of hydroxyskatole
and mauve factor excretion in schizophrenia. Int J Neuropsychiatry 3: 327-
331.
18. McGinnis WR, Audhya T, Walsh WJ, Jackson JA, McLaren-Howard J (2008)
Discerning the mauve factor, Part 1. Altern Ther Health Med 14: 46-62.
19. Mikirova NA, Casciari JJ, Hunninghake RE (2013) The Orthomolecular
Correction of Metabolic Imbalances Found in Attention Deficit Hyperactivity
Disorder: A Retrospective Analysis in an Outpatient Clinic. J Orthomolecular
Medicine 28: 1-10.
20. Cutler MG, Graham DJ, Moore MR (1990) The mauve factor of porphyria,
3-ethyl-5-hydroxy-4,5-dimethyl-delta-3-pyrroline-2-one: effects on behaviour
of rats and mice. Pharmacol Toxicol 66: 66-68.
21. Moore MR, Graham DJ (1980) Monopyrroles in porphyria, psychosis and
lead exposure. Int J Biochem 12: 827-832.
22. Genter St Clair MB, Amarnath V, Moody MA, Anthony DC, Anderson CW,
et al. (1988) Pyrrole oxidation and protein cross-linking as necessary steps
in the development of gamma-diketone neuropathy. Chem Res Toxicol 1:
179-185.