The Role of Mitochondria in Cancer and Other Chronic Diseases
The Role of Mitochondria in Cancer and Other Chronic Diseases
Dorothy D Zeviar, Michael J Gonzalez, Jorge R Miranda Massari, Jorge Duconge, Nina Mikirova
Journal of Orthomolecular Medicine, 2014, Vol 29, No 4: 157-166
Nutrition is the foundation and basis of good health; therefore, it stands to reason that a proper diet would assist in the prevention of common 21st century chronic diseases such as heart disease, diabetes, neurodegenerative diseases, and cancer. In this article we explain the roles of mitochondria in health, and the biochemistry of mitochondria in degenerative disease. We examine the role of oxygen in both (aerobic) oxidative phosphorylation (OxPhos) and (anaerobic) glycolysis, and how the latter may contribute to chronic disease states. We discuss the biochemical mechanisms behind adenosine triphosphate production and the simultaneous production of Reactive Oxygen Species (ROS) (free radicals), and the chronic effects of cellular ROS damage. Lastly, we discuss the cellular health-enhancing effects of reductive molecules (antioxidants) and an alkaline environment, and how this contrasts with an acidic environment/ diet, which contributes to chronic disease and the pathological state.
Mitochondrial Basics
Mitochondria serve several important
cellular roles, but first, we shall discuss some
background history, structure and the roles
mitochondria play in cellular health. It is
generally recognized and agreed that mitochondria originated from an aerobic bacteria
approximately 1-3 billion years ago, which
merged with a pre-existing unicellular organism. Both organisms developed a symbiotic
relationship which provided a way to create
aerobic cellular respiration and produce much
more energy. This in turn, supports the development of complex multi-cellular aerobic
organisms. Mitochondria are the only subcellular organelle/organism with their own
mtDNA.1
Because mtDNA is maternally transmitted by the ovum at conception (inherited from
one’s mother), its genetic defects or variants,
deficiencies (if any) are limited to the mitochondria; the cellular-nuclear DNA (nDNA)
is governed by Mendelian inheritance principles. In contrast to nDNA which is made up
of 3.3 billion base pairs (bp) of genes, mtDNA
is circular and composed of 16,569 bp. These
bp include 37 genes, of which 24 encode for
mitochondrial translation and 13 encode for
the cellular respiratory chain.2
nDNA is protected by histones which shield nDNA from
free radical damage, however, mtDNA is
not protected by histones, so they are more
susceptible to oxidative damage.3
mtDNA
may generate up to 10 times the number of nDNA mutations for two reasons – mtDNA
resides close to the electronic transport system
(ETS) inside the inner mitochondrial membrane and mtDNA lacks repair mechanisms,
so once damaged, the mitochondria may be
slated for apoptosis.4
Mitochondria Structure and Roles
The number of mitochondria per cell is
energy/function dependent; i.e., those cells
that require and expend the most energy
contain the highest number of mitochondria.
Most cells have between a few hundred to
over 20,000 mitochondria; they are concentrated most heavily in cells of the heart, brain,
liver, muscles, gastrointestinal tract, and kidneys.5
Mitochondria are composed of two
membranes. The more porous outer membrane contains porin and allows molecules
up to approximately 10 kDa to freely diffuse
across the membrane. The inner, more tightly
constructed (less permeable) membrane contains cardiolipin, a phospholipid which has
both a higher affinity for inner membrane
proteins, and, having two unsaturated bonds,
is more susceptible to oxidative damage.
Components of the electron transport system
(ETS) are found along the inner membrane.
The space between the two membranes is the
intermembrane space where Cytochrome c is
found. Inside the inner membrane is the mitochondrial matrix which contains many of
the enzymes necessary for adenosine triphosphate (ATP) production (enzymes associated
with the Kreb’s Cycle), as well as the mitochondrial genome.6
Mitochondria play many important roles
in human biology, including synthesis of
heme, lipids, amino acids and nucleotides. As
mentioned above, they are involved in initiating cellular apoptosis. Their most important
role, however, is the production of ATP. Mitochondria generate 95% of the ATP in the
cell, and rely on ATP for its own functions.7, 8
Due to the location of the ETS adjacent
to the inner mitochondrial membrane, the
generation of free radicals as a normal part
of oxidative phosphorylation (production of
ATP), as well as the lack of histone protection for mtDNA, much oxidative damage can
occur to mitochondria, and indeed does occur
in normal physiological reactions as well as in
chronic disease. Later, we will discuss the role
that an alkaline diet can play in preventing
much of this oxidative damage.
Review of ATP Biosynthesis
As stated above, the primary role of mitochondria is to synthesize ATP (cellular energy). This process is also known as cellular
respiration. As humans, we derive all our energy from the food we eat, which the mitochondria metabolize into glucose, amino acids and fatty acids. Because we derive all our
cellular energy from the food we eat, this fact
emphasizes the point that eating whole food
is necessary for proper ATP production and
general cellular functions. Recent research has
linked all chronic disease, including cancer, to
deficiencies in mitochondrial structure and
function.1
The Role of Oxygen in Both (Aerobic)
Oxidative Phosphorylation and (Anaerobic) Glycolysis
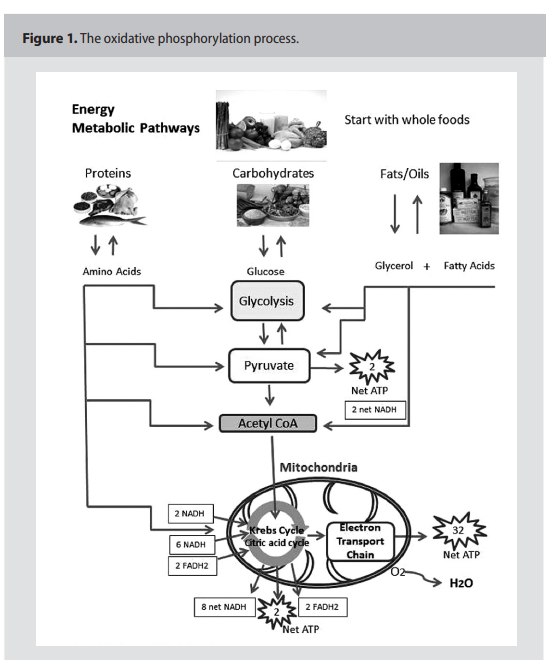
As presented in Figure 1, (p.159) the
“normal” process of oxidative phosphorylation (OxPhos) creates approximately 38
ATPs per glucose molecule and approximately 90% of the cell’s energy requirements.
Under normal aerobic conditions, pyruvate is
oxidized by NAD+ and a dehydrogenase enzyme that converts pyruvate to Acetyl-CoA
and CO2. This reaction requires oxygen to
oxidize NADH back to NAD+ to continue
the metabolic process.9
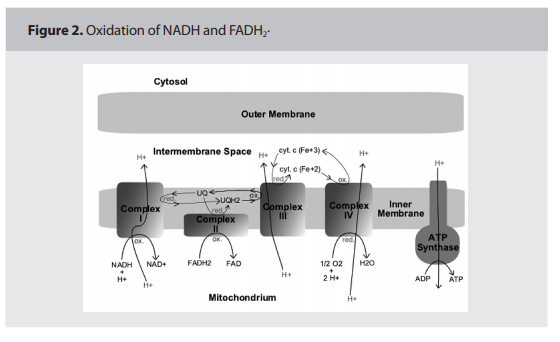
This section will provide a simplified explanation of the ETS and OxPhos in the inner membrane of the mitochondria. Research
has identified five protein complexes on the
inner mitochondrial membrane related to the
ETS and OxPhos processes; Complexes I, II,
III, IV are part of the ETC, and Complex V
is where OxPhos or the conversion of ADP
to ATP actually takes place. This process requires co-factors that actually carry the electrons “down” the ETS such as cytochrome C
and Co-Q, as illustrated in Figure 2. (p.160)
The entire process is actually one of oxidation
of NADH and FADH2, by-products of the
Kreb’s Cycle, to H2O. 10
Complexes I, III, and IV “pump” protons
from the inner membrane across the membrane into the intermembrane space, creating a “proton gradient” that is necessary for
ATPase conversion of ADP to ATP (phosphorylation). The potential of hepatocytes,
for example, has been measured at 170mV,
but the normal cell potential is 50-70 mV. A
proton gradient is necessary for efficient ETS
function. The combination of movement of
protons “down” the ETS and the phosphorylation of ADP in Complex V is called the
coupling of cellular respiration with the synthesis of ATP. It is said that the efficiency with which foods are metabolized and converted
to energy is determined by the efficiency of
this “coupling” process. It is estimated that
each complex pumps four protons across the
membrane.10,11 The “pumping” of protons into
the intermembrane space helps maintain an
alkaline pH inside the mitochondria, which
then creates a negative potential with respect
to the cytosol.11 Acidic substances, xenobiotics, and drugs can also “uncouple” the ETS
from OxPhos. As previously stated, the entire
ETS and OxPhos process produces approximately 38 ATP.
Because ATP production occurs in the
cristae of the inner membrane, close to the
ETS where protons are “pumped” and occasionally “lost,” the mitochondria are subject
to great oxidative damage themselves by their
own processes. Although aerobic OxPhos is
the optimal process for producing ATP, it
is not without inherent danger to the mitochondria themselves, as it also produces ROS
such as the superoxide radical O2.-, hydrogen
peroxide H2O2, the hydroxyl radical HO. ,the
perhydroxyl radical HO2. , and peroxynitrite
ONOO- . During normal OxPhos, 0.4 – 4%
of all oxygen consumed is converted in mitochondria to superoxide O2.-.1
These ROS
contribute to enzymatic damage, membrane
damage and subsequent apoptosis. Not only
do ROS accumulate with age, but they negatively affect mtDNA replication and repair
processes. Organelles that have sustained
damage to their DNA, membranes, or respiratory chain (ATP synthesis) proteins will
suffer from a chronic energy shortage and
diminished or nonexistent proton gradient.12
Defective mitochondria accumulate most in
ATP-active organs such as the brain, heart
and muscle, which may partly explain the increasing incidence of chronic diseases involving these organs. These ROS can be “paired”
and neutralized in the cell with a diet high in
antioxidants, found in a typical alkaline diet
rich of fresh fruits and vegetables.
Anaerobic Glycolysis
This discussion about ROS damage to
mitochondria relates to anaerobic glycolysis.
From the point in the metabolic pathway
where pyruvate metabolizes to Acetyl-CoA,
pyruvate can also take another form as lactate (C3H5O3-) under conditions of low
oxygen. The attention should be directed to
the one-way arrow emerging from pyruvate
(C3H4O3) to Acetyl-CoA (C21H36N7O16P3S)
to indicate that, at this point in the metabolic
process, pyruvate can only metabolize to
Acetyl-CoA in the presence of oxygen. When
muscles have over-exercised and “used up” the
available oxygen, pyruvate cannot convert to
Acetyl-CoA and instead turns to lactic acid
(C3H6O3). This phenomenon occurs during heavy exercise or stress, for example, but
can also occur in the initial stages of cancer
and other degenerative and chronic diseases
which affect cellular integrity. Lactic acid creates an acidic cellular environment that, if
not immediately corrected, contributes to a
chronic acidic cellular environment which is
conducive to cellular breakdown, loss of function and predisposition to cancer. The usual
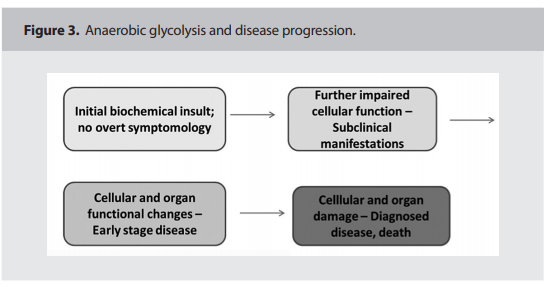
disease progression may follow the pattern
depicted in Figure 3. (below)13
Recall that mtDNA lacks protective
histones and repair mechanisms; therefore,
they are more susceptible to oxidative damage. And although each cell contains numerous mitochondria (from hundreds to over
20,000), one would think that occasional mitochondrial damage would not significantly
impact a cell or an organ. And occasional mitochondrial damage does not affect cellular or
organ function. However, years of cumulative
oxidative damage to both mtDNA and subsequently nDNA does indeed adversely affect
cellular and organ function which leads to
disease states, cancer and aging.
ROS Damage
As stated above, a two-edged sword regarding ATP production is the simultaneous production of necessary ROS that may
have a role in gene regulation and excessive
damaging ROS leading to the disease state,
under both aerobic and anaerobic conditions.
One explanation for how ROS damage contributes to chronic disease conditions is that
excess calories (or poor quality calories), and
the lack or excess of exercise generate more
electrons than the ETS can handle, leaving
more electrons in the inner membrane space
(because they can’t be pumped back out into
the intermembrane space). This adversely affects the proton gradient necessary for ADP
coupling with P to create ATP, stalling the
ETS process. Additionally, with less oxygen
available to pair with the protons created in
the ETS process, the cells cannot make H2O
as a by-product of OxPhos, so more ROS
accumulate in the cells, contributing further
to mtDNA damage and subsequent nDNA
damage.14 ROS contributes to mtDNA damage/deletions/mutations, and as less ATP
is produced and cellular functions diminish,
subsequent replicated mitochondria become
less and less robust and unable to successfully
carry out cellular and organ functions, thus
contributing to chronic degenerative disease.
Apoptosis
Recall that another important role of mitochondria is that of regulating cellular apoptosis. Because ROS damage negatively impacts
ATP production, necessary for ALL cellular
functions, regulation of apoptosis is also affected. Apoptosis is the process of programmed
cell death, necessary for the renewal of all body
cells, and for the continuity of life. Approximately 30-50 billion cells are replaced daily
in the average human. 15 However, too much
apoptosis can cause muscle and organ failure,
and too little may contribute to tumorigenesis.
Recall that the mitochondrial inner
membrane is composed primarily of cardiolipin, an easily oxidized phospholipid. When
the mitochondrial membrane is damaged
(due to any of the stressors mentioned above),
apoptotic signals are released which cleave
to nDNA and initiate cell death. The more
membrane damage, the more rapid cellular degradation occurs. Several proteins and
other substances in the mitochondria initiate
apoptosis. During this process, cytochrome
C is released from the intermembrane space
into the cytosol which causes cell death (after
other substances are triggered and released).
So although 30-50 billion cells are replaced
daily, if apoptosis in one body system is greater than the number of cells replaced, systemic
disease and/or organ failure ensues. As cells
continue to die off through apoptosis, tissue
function decreases, which eventually lead to
symptoms and chronic degenerative disease
(refer to boxes 2 and 3 in Figure 3).13
Chronic Disease, Cancer and
Mitochondria
As discussed earlier, lacking histones and
with lowered ATP production, mitochondria
have limited ways of self-repair once damage
from ROS has been inflicted. In this situation, cells cannot even make the RNA and
DNA they require to function without mitochondria. When mtDNA becomes damaged,
it is more difficult to copy accurately, resulting
in errors of transcription, deletions and mutations. Oxidation from ROS results in a series
of cellular insults: cell membranes lose their
integrity, the proton gradient is diminished
causing less ATP to be produced, cellular
proteins necessary for all other cellular functions unfold and lose their affinity for their
enzymes, and cytochrome C is released into
the cytosol stimulating apoptosis, all in a continuous feed-forward cycle of cellular, tissue
and organ dysfunction (chronic degenerative
disease). Production of ATP is the key differentiator and chief purpose of mitochondria in
the cell; they are the keystone to proper tissue
and organ function and even gene regulation
in humans. This point cannot be over-stated
or over-emphasized; without fully functioning mitochondria, we cease to exist. Research
is finding that cancer cells also exhibit increased mitochondrial damage by ROS.9
As discussed above, ROS impedes the ETS,
resulting in not only reduced production of
ATP, but an excess of unoxidized NADH and
pyruvate, which in turn get reduced to lactate.
Additionally, high ROS concentrations permit histone acetylation to predominate, which
accelerates (faulty) nuclear transcription and
thus replication, and initiates the release of
NFkB into the nucleus (a significant proinflammatory cytokine which also damages
nDNA). At the same time, however, cell differentiation and apoptosis signals are silenced
with histone acetylation, eventually resulting
in over-replication favoring tumorigenesis. 16
Gonzalez et al further explained the connection between dysfunction in the ETS
and apoptosis: more CO is produced as a
by-product of inefficient cellular respiration,
which also blocks apoptosis. Cancer cells
have a lower proton gradient: only -15 mV
compared to a normal cell of 50-70 mV. A reduced gradient simultaneously reduces ATP
output. Complicating this metabolic scenario
is the fact that without sufficient ATP, cells
lose their ability for cell-to-cell communication, so as “individualized” unicellular cells,
must form colonies to survive, forming what
we know as the tumor. Thus, cancer is a cell
survival mechanism in a hostile (acidic) environment, since cancer cells have a hard time
surviving in an alkaline environment.16 ROS
contributes both to chronic disease manifestation through the mechanisms of mitochondrial dysfunction and subsequent tissue/ or
gan loss of function; as well as tumorigenesis
progression through the mechanisms of uncontrolled nDNA replication without differentiation. Cancer cells appear to thrive under
anaerobic conditions; this phenomenon was
first observed by Warburg in the 1930s.
Early History of Cancer Research –
Warburg, Szent-Gyorgyi, and Pauling
According to the CDC, cancer (all forms)
is now the second-leading cause of mortality
among people in the developed world, exceeded only by heart disease.17 By its nature
and characteristics, cancer is the uncontrolled
overgrowth of cells which we call a tumor.
Normal cellular functions initiated by the
mitochondria such as apoptosis, and cellular
division/ replication are dysfunctional in a
cancerous environment, due to loss of cellular
membrane integrity, and an increasing acidic
cellular environment, as stated above. When
cellular functions no longer operate properly,
the cell accumulates ROS and lactate, leaving
the cell to depend on anaerobic glycolysis for
energy, which generates only two ATPs.10
Our experience has revealed that conventional oncology believes that a significant
proportion of cancers are the result of genetics, yet recent statistics inform us that genetics play a role in only 5% of cases.18 We now
know that mitochondrial activity/ function
determines whether oncogenes get “switched
on” or “off;” an alkaline diet appears to help
keep these genes under control.
Otto Warburg, a German biochemist, was
a pioneer in observing and publishing research
into cellular respiration and the effects on
cancerous cells/ tumor growth; he was awarded the Nobel Prize in Physiology in 1931 for
his work. His research concluded that, unlike
normal cells which depend on aerobic oxidative phosphorylation to produce ATP, cancerous cells instead use anaerobic respiration for
energy production. As he wrote and lectured,
“The prime cause of cancer is the replacement
of the respiration of oxygen in normal cells
with the fermentation of sugar. All normal
cells meet their energy needs by respiration of
oxygen, whereas cancer cells meet their energy needs in great part by fermentation. Thus,
cancer cells are partial anaerobes.” He added,
“During cancer development aerobic respiration fails, fermentation appears, and highly
differentiated cells are transformed into fermenting anaerobes, which retain only the now
useless property of growth.” He concludes,
“Cancer is ultimately a problem of how cells
use or misuse oxygen to burn sugars.”19 Sadly,
this theory was discounted by the mainstream
medical establishment, continued to be discounted throughout the 1960s when Warburg lectured internationally, and continues
to be ignored by today’s oncologists who refute the role of anaerobic glycolysis, sugar and
ROS in the creation of cancerous conditions.
Research done by Vaughn and Deshmukh
demonstrate that it is “glucose metabolism
which protects cancer cells from cytochrome
C mediated apoptosis.”20 Albert Szent-Gyorgyi, who won the Nobel Prize in Physiology
in 1937 for his work in discovering vitamin
C elucidated what was the theory of cellular
combustion (producing energy), i.e., that “the
combustion of hydrogen is the real energysupplying reaction.”25 Empirical experimentation with Hungarian paprika and lemons
had a therapeutic effect on colleagues with
damaged capillary blood vessels; the positive
effect of vitamin C on blood vessel integrity
and wound-healing is well-documented. Vitamin C is also a powerful RedOx agent and
co-factor in many enzymatic reactions.21
Following on the research of Warburg
and Szent-Gyorgyi, in the 1970s Linus Pauling conducted empirical studies of both oral
and IV vitamin C on people with cancer and
the common cold, reasoning that vitamin C
therapy increased survival of cancer patients
by four times compared to control groups.
He co-wrote a book entitled “Vitamin C and
Cancer” and with a colleague Ewan Cameron,
but was still labeled a “quack” by the medical establishment. Gonzalez et al wrote that
ascorbate (vitamin C) may preferentially target the mitochondria by increasing electron
flux, thus increasing the production of ATP
and thus, the “normalization” of the apoptosis
function. They added that a greater amount of
vitamin C optimizes the production of ATP
as well as cell-to-cell communication and cell important with regards to both the dosage of
vitamin C as well as the timing of application
during oncologic therapies, as Vitamin C can
have both antioxidant and pro-oxidant characteristics.22 RedOx therapy may become the
“medicine of the 21st century.
A recurring theme is that mainstream allopathic oncologists continue to deny the efficacy of vitamins, minerals, whole foods and
antioxidants on prevention and treatment
of chronic degenerative diseases and cancer.
With the overview of biochemical processes
involved in mitochondrial and cellular dysfunction as outlined in this paper, the evidence
appears to be strong that an alkaline diet high
in antioxidants (fruits and vegetables) would
help prevent chronic degenerative disease and
cancer, and lead to a better quality of life.
The Protective, Preventive Action of
an Alkaline Diet
Prevention of cancer involves two elements: consumption of the proper diet and
the avoidance of substances that damage the
mitochondria. Damage to mitochondria is
known to have a key role in the pathogenesis
of an extensive amount of disorders such as
schizophrenia, dementia, Alzheimer’s disease,
epilepsy, strokes, neuropathic pain, Parkinson’s
disease, ataxia, transient ischemic attack, cardiomyopathy, coronary artery disease, chronic
fatigue syndrome, fibromyalgia and diabetes
among others. A proper diet must include
sufficient nutrients to sustain efficient aerobic
respiration. This includes the macronutrients
that are the energy and macromolecules for
functional and structure and function and the
micronutrients that facilitate efficient functioning of the biochemical pathways to extract
and transform energy into a biologically useful form. These micronutrients include the Bcomplex, various minerals, other cofactors such
as CoQ10, lipoic acid and acetyl L carnitine and
the electrolyte balance to promote the conditions for an efficient physiological functioning.
Risk factors linked with chronic diseases
(e.g., cancer, lung diseases), such as stress, tobacco, environmental pollutants, radiation, infection, cause damage to cells through excessive or uncontrolled generation of ROS.23
Xenobiotics that damage mitochondrial
membrane include environmental toxicants
and medications. Tobacco smoke reduces
arterial oxygenation and increases oxidative
stress and decreases cytochrome oxidase in
complex IV if the mitochondria, 25% after 30
minutes of passive smoke and the enzyme activity continues to decrease with time.24
Because the mitochondria is crucial in
energy production, the mitochondrial dysfunction can be related to various groups of
diseases including the main killers in our
society cancer and cardiovascular disease.25
Other environmental factors include some
insecticides and pesticides and fat soluble
chemicals with benzene rings such as hair dye
and paint fumes.
Research has demonstrated that medications are a major cause of mitochondrial
damage, which may explain many adverse
effects. These offenders include psychotropic
drugs, anticonvulsants, anti-cholesterol medications, analgesics and anti-inflammatory
agents, antibiotics, steroids, anticancer chemotherapy, Diabetes medications and HIV/
AIDS medications. While certain nutritional
cofactors might limit the damage caused to
mitochondria by medications, there is still
much research needed in this area.26
Chronic inflammation can stimulate
all stages of tumorigenesis, (DNA damage,
uncontrolled replication, inhibition of apoptosis, augmented angiogenesis and tissue invasion/metastasis. Chronic inflammation is
prompted by environmental factors (e.g., infection, tobacco, asbestos) and host gene mutations factors (e.g., Ras, Myc, p53). Despite
the extensive research published over the last
decade, many of the precise molecular mechanisms are still in elucidation and discussion.
It has been proposed that activation of
Ras, Myc, and p53 cause mitochondrial dysfunction, which then causes mitochondrial reactive oxygen species (ROS) production and
consequent signaling transcription factors
(eg, NFkappaB, STAT3, etc.) that promote inflammation-associated cancer.27 However, the
bioenergetic theory of carcinogenesis28 proposes that mitochondrial dysfunction could be the original insult that induces signaling
that activates the oncogenes and transcription
factors. Inflammation-associated cancers produced from signaling from the mitochondrial
are being identified that may prove useful for
developing innovative strategies for both cancer prevention and cancer treatment.
Diet and Biochemical Conditions
Neustadt suggests that because the major
reason and root cause for mitochondrial dysfunction (and thus chronic disease and cancer)
lies in a surplus of ROS that cannot effectively
be neutralized, that RedOx therapy (IV vitamin C, alkaline diet, supplements, enzymes,
etc) may be a viable lifestyle option for both
prevention and treatment. Because research is
still lacking in the dosage and timing of reductive therapy, the best way to determine vitamin and supplement needs is through urinary
organic acid testing. Optimal mitochondrial
function is dependent upon sufficient vitamins, minerals, enzymes, co-factors and all the
nutrients necessary for optimal cellular function, all of which are found in a good alkaline
diet.1
Cancer cannot exist in an alkaline, oxygen-rich environment. To overcome cancer,
we must change our internal environment.9
This is the mitochondrial correction concept.
The co-factors necessary for complete
Kreb’s Cycle metabolism include cysteine, sulfur, iron, magnesium, manganese, lipoic acid,
niacin, thiamin, riboflavin, and pantothenic
acid, the last four of which are in the Vitamin
B family. Supplementation with lipoic acid
and acetyl-L-carnitine can improve mitochondrial function.29 Carnitine is necessary to
move Acetyl-CoA into the mitochondria with
vitamin C as a co-factor. The ETS requires
both CoQ10 and flavins which include riboflavin, iron-sulfur complexes, copper and heme
molecules. Heme synthesis requires pyridoxine (B6), riboflavin (B2), iron, copper, and zinc.
Glutathione is a major anti-oxidant which requires selenium as a co-factor for production.
Deficiencies in any of these substances can
cause increased ROS production and loss of
cellular function. Antioxidant herbs and supplements include such substances as turmeric
(curcumin), green tea, resveratrol, and garden
herbs such as oregano. Anti-inflammatory
substances include Omega-3 fish oil, flax oil,
vitamin E, boswellia, and ginger.1
Alkaline vs Acidic Environment
Average adult humans eating Western diets have chronic, low-grade metabolic acidosis
at a grade that can be estimated by the net rate
of endogenous non-carbonic acid production
(NEAP), which varies with diet. 30 Some agerelated problems such as bone mass decline,
osteoporosis, and decrease in muscle mass.
Chronic, low-grade is in part caused by diet-dependent acidosis and may therefore be
improved by diet modification and/or supplementation.
Our current “Standard American Diet”
(SAD) is acidic, made so by over-consumption
of high-glycemic foods, processed foods, sugar,
meats, coffee and alcohol, and anything made
with white flour. Stress and toxins also contribute to an acidic environment. Mitochondrial
enzymes in the matrix work best in an alkaline
environment, thus optimizing their metabolic
processes.31,32 According to Gonzalez, alkaline
solutions absorb oxygen, whereas acidic environments expel oxygen, which explains why
anaerobic organisms thrive in an acidic environment, and why tumorigenesis is also favored in an acidic environment. A lowered pH
contributes to a lowered membrane potential
which results in cellular dysfunction and lowered ATP production, again, favoring chronic
disease progression and carcinogenesis.16
The ideal blood pH range is 7.35 to 7.45,
with the majority of holistic health practitioners preferring the higher range, closer to
7.4. One of the chief ways the body creates
homeostasis is to “steal” minerals from bones
and other vital organs. This compensating
mechanism, of course, contributes to loss of
vital co-factors involved in important enzymatic reactions, which in turn decreases cellular and organ function eventually leading to
chronic disease and/or cancer.
Concluding Remarks
Developing a healthy lifestyle, with an
emphasis on increasing vegetables in the diet,
would decrease ROS and provide the or ganism with a balance of nutrients that fosters a healthy biochemical environment that
strengthens the composition and function of
the mitochondria should be protective against
chronic diseases such as cancer.
Competing Interests
The authors of this report declare that
they have no competing interests.
References
1. Neustadt J, Pieczenik S: Mitochondrial dysfunction
and molecular pathways of disease. Exp Molec
Pathol, 2006; 83: 84-92.
2. Birch-Machin M: The role of mitochondria in ageing
and carcinogenesis. Clin Exp Dermatol, 2006; 31:
548-552.
3. Alexeyev MF: Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J,
2009; 276(20): 5768–5787.
4. Cohen B, Gold D: Mitochondrial cytopathy in adults:
What we know so far. Cleveland Clin J Med, 2001;
68: 625-642.
5. Alberts B, Johnson A, Lewis J, et al: Molecular Biology of the Cell. New York: Garland Publishing Inc.
1994; 32-36.
6. King A, Selak MA, Gottlieb E: Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene, 2006; 25:
4675-4682.
7. Pham T, Loiselle D, Power A, Hickey AJ: Mitochondrial inefficiencies and anoxic ATP hydrolysis
capacities in diabetic rat heart. Am J Physiol Cell
Physiol, 2014; 307: C499-C507.
8. Hüttemann M, Lee I, Grossman LI, et al: Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny:
respiration, apoptosis, and human disease. Adv Exp
Med Biol, 2012; 748: 237-264.
9. Clark D: Basic Science Aspects of the Mitochondria.
Section X: Cancer, 2000; p.2,6. Retrieved from:
[http://healingtools.tripod.com/primecause1.html/]
10. Kowald A: The mitochondrial theory of aging: Do
damaged mitochondria accumulate by delayed
degradation? Exp Gerontol, 1999; 34: 605-612.
11. Neustad J, Pieczenik S: Foundations and Applications
of Medical Biochemistry in Clinical Practice. Bloomington, IN. iUniverse. 2009.
12. Wallace D: A mitochondrial paradigm of metabolic and degenerative diseases, aging and cancer:
A dawn for evolutionary medicine. Ann Rev Genet,
2005; 39:359.
13. Karam J: Apoptosis in Carcinogenesis and Chemotherapy. Netherlands: Springer. 2009.
14. Belikova N, Vladimirov YA, Osipov AN, et al: Peroxidase activity and structural transitions of cytochrome C bound to cardiolipin-containing membranes. Biochem, 2006; 45: 4998-5009.
15. Birch-Machin M: The role of mitochondria in ageing and carcinogenesis. Clin Exper Dermatol, 2006;
31: 548-552.
16. Gonzalez MJ, Rosario-Pérez G, Guzmán AM, et al:
Mitochondria, energy and cancer: The relationship
with Ascorbic acid. J Orthomol Med, 2010; 25: 29-38.
17. CDC Centers for Disease Control and Prevention.
Cancer prevention and control. Cancer statistics by
cancer type. Retrieved from [http://www.cdc.gov/
cancer/dcpc/data/types.htm].
18. Seyfried TN, Shelton LM: Cancer as a metabolic
disease. Nutr Metab (Lond), 2010; 7: 7.
19. Warburg O: On the Origin of Cancer Cells. Am Assoc Adv Sci, 1956; 123: 309-314.
20. Vaughn AE, Deshmukh M: Glucose metabolism
inhibits apoptosis in neurons and cancer cells by
redox inactivation of cytochrome c. Nat Cell Biol,
2008; 10: 1477-1483.
21. Szent-Gyorgyi A: The living state and cancer. Proc
Natl Acad Sci USA, 1977; 74: 2844-2847.
22. Padayatty S, Katz A, Wang Y, et al: Vitamin C as an
antioxidant: evaluation of its role in disease prevention. J Am Nutr, 2003; 22: 18-35.
23. Gupta SC, Hevia D, Patchva S, et al: Upsides and
downsides of reactive oxygen species for cancer:
the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal,
2012; 16: 1295-1322.
24. Gvozdjak J, Gvozdjakova A, Kucharska J, Bada V:
The effect of smoking on myocardial metabolism.
Czech Med, 1987; 10: 47-53.
25. Yang Z, Harrison CM, Chuang GC, Ballinger SW:
The role of tobacco smoke induced mitochondrial
damage in vascular dysfunction and atherosclerosis.
Mutat Res, 2007; 621(1-2): 61-74.
26. Neustadt J, Pieczenik SR: Medication-induced mitochondrial damage and disease. Mol Nutr Food
Res, 2008; 52: 780-788.
27. Kamp DW, Shacter E, Weitzman SA: Chronic inflammation and cancer: the role of the mitochondria. Oncology, 2011; 25: 400-410.
28. Gonzalez MJ, Miranda Massari JR, et al: The bioenergetic theory of carcinogenesis. Med Hypotheses, 2012; 79: 433-439.
29. Hagen TM, Moreau R, Suh JH, Visioli F: Mitochondrial decay in the aging rat heart: evidence
for improvement by dietary supplementation with
acetyl-L-carnitine and/or lipoic acid. Ann NY Acad
Sci, 2002; 959: 491-507.
30. Frassetto LA, Todd KM, Morris RC Jr, Sebastian
A: Estimation of net endogenous noncarbonic acid
production in humans from diet potassium and protein contents. Am J Clin Nutr, 1998; 68: 576-583.
31. Hansen SH, Andersen ML, Cornett C, et al: A role
for taurine in mitochondrial function. J Biomed Sci,
2010; 17(Suppl 1): S23.
32. Minich D, Bland J: Acid-alkaline balance: Role
in chronic disease and detoxification. Altern Ther
Health Med, 2007; 13: 62-65.